AbstractObjectiveUnilateral biportal endoscopic lumbar discectomy and microscopic lumbar discectomy are typical surgical treatments for spinal disease that are performed in different environment. This study investigated differences in bone healing at the postoperative laminecrtomy site between 2 surgical treatments performed in different environments.
MethodsFrom January 2018 to June 2021, 66 patients who underwent laminectomy at Department of Neurosurgery, Gangnam Severance Hospital were retrospectively reviewed. All patients were matched for sex, age, body mass index, bone mineral density, and follow-up duration at a 1:2 matching ratio and were divided into the UBE group (22 patients) and the microscopic discectomy group (44 patients). We investigated the site of laminectomy shown on preoperative and postoperative x-ray images using ImageJ software. The factors related to bone healing were also investigated.
ResultsThe average bone healing area was 69.59 mm2 in the UBE group and 44.56 mm2 in the microscopic discectomy group, constituting a significant difference (p=0.022). The remaining laminectomy area was significantly lower in the UBE group than in the microscopic discectomy group (13.91 mm2 vs. 53.84 mm2, p<0.001). The bone recovery ratio in the UBE group was 85.42%, compared to 51.33% in the microscopic discectomy group, which was a significant difference (p<0.001). The primary laminectomy area, bone healing during 6 months, and clinical outcomes were not significantly different between the 2 groups.
INTRODUCTIONDecompressive laminectomy is a treatment option for symptomatic spinal stenosis, herniated lumbar disc, and other degenerative lumbar disease. In 1829, AG Smith performed the first laminectomy [1]. The current gold standard treatment for lumbar disc herniation refractory to conservative treatment is facet-preserving partial hemilaminectomy and discectomy [2]. Decompression through extensive laminectomy with facet violation or excessive laminectomy has a potential risk of future instability and deformity [3]. The degree of bony resection required to achieve effective neural decompression with minimal bony damage and reduced risks of the aforementioned complications remains debatable. Nevertheless, normal structure-preserving surgery is important, and various surgical methods have been developed to achieve neural decompression and discectomy.
At the beginning of the 21st century, several authors introduced various lumbar decompression and discectomy techniques to preserve the posterior midline structures [4-6]. Such techniques evolved into microendoscopic decompression and discectomy with the use of microscopy and tubular retractors [7]. Recently, several authors have used unilateral biportal endoscopic (UBE) discectomy for treatment of lumbar degenerative disease [8-15]. UBE discectomy requires less extensive bone resection, limits muscle damage, can yield sufficient decompression despite minimal neural retraction [16,17], and can be performed under highly magnified views. Decompression of the contralateral traversing root is relatively easy due to the wide angle available for insertion of an arthroscope. With these advantages, the UBE technique has developed and been applied in the cervical, thoracic, and lumbar spine [18-21].
Microscopic lumbar discectomy, in which a midline incision is made with unilateral dissection of the paraspinal muscles for exposing the bony spinal structures, is performed in an air environment. The main difference from microscopic open surgery is that UBE discectomy is performed in a water environment. Continuous saline irrigation through the portals during surgery prevents temperature increase of the bone surface and reduces damage of the bone. The difference between microscopic lumbar discectomy and UBE discectomy is the environment in which surgery is performed. Although there have been many prior studies comparing these 2 surgeries, none has compared them in terms of laminectomy area and healing. Therefore, we studied to investigate the difference of bone healing and preservation in water-based UBE discectomy and air-based microscopic lumbar discectomy.
MATERIALS AND METHODS1. Study PatientsThis study is a retrospective analysis of patients who underwent UBE or microscopic lumbar discectomy to treat back pain or radiculopathy due to degenerative lumbar disease between January 2018 to June 2021, at a single institute. The operative procedures were determined according to the experience and preferences of the operating surgeons. All included patients met the following criteria: (1) age > 18 years; (2) at least 1-year postoperative day (POD); (3) outpatient follow-up (F/U) duration of at least 1 year; (4) sufficient imaging data including pre- and postoperative x-rays; (5) no revision surgery during follow-up; and (6) adequate image quality to calculate laminectomy area. Patients with tumors, infection, trauma, or congenital bone deformities such as spina bifida were excluded (Figure 1). This study was approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital, Yonsei University College of Medicine (IRB No. 3-2022-0084).
2. Surgical ProcedureThe patient is positioned prone over the radiolucent Wilson frame during under general anesthesia. For UBE discectomy, 2 incisions are placed approximately 3 cm apart, with the center of each placed on a target disc space. After inserting a serial dilator into the working portal, a radiofrequency ablation probe was used to dissect the paraspinal muscle. UBE discectomy is performed under continuous normal saline irrigation (water-based). It is critical to ensure that the final layer of draping is waterproof and that there is a smooth drainage system for saline outflow. After paraspinal soft tissue is dissected, the lower lamina of the upper lumbar spine and upper lamina of the lower lumbar spine are partially removed via an automated drill and Kerrison punches until the ligamentum flavum is released from the bony structures. After bone work is complete, the ligamentum flavum was dissected and removed using Kerrison punches. Next, the exiting nerve root is identified and disc fragments or osteophytes removed [22]. Decompressed root confirmation and disc space exploration are performed. The skin is sutured using nonabsorbable 3-0 sutures (Nylon, Coviden, Minneapolis, MN, USA).
Microscopic lumbar discectomy is performed under general anesthesia. The surgical procedure follows the standard method using a Caspar, or Taylor retractor system [23,24]. A 2- to 3-cm midline skin incision is made, and the subcutaneous tissue is dissected down to the level of the lumbar fascia to expose bony structures and interlaminar ligamentum flavum (air-based). The inferior portion of the superior lamina is removed with a high-speed drill and Kerrison punches to properly expose the herniated disc and neural elements. Then, the ligamentum flavum is removed for disc discrimination and the nerve roots were exposed. Any protruded disc area is removed, and the mobility of the root is assessed using a hook dissector. Wound closure was performed using 1, 2-0 absorbable sutures (Vycryl, Ehicon, Somerville, NJ, USA) and a skin stapler.
3. Data CollectionInformation regarding patient characteristics, including age, sex, smoking, height, weight, body mass index (BMI), bone mineral density (BMD), and clinical symptoms, was collected. Furthermore, type of the surgery, operating time, hospital stay, estimated blood loss (EBL), and F/U duration were documented. Surgeons collected the following information for each patient preoperatively, postoperatively, and at the 1-month, 6-month, 12-month, 18-month, 24-month, and final F/Us. Laminectomy area, healing area, remaining laminectomy area (laminectomy area minus bone healing area), bone recovery rate (ratio of healing area to laminectomy area), and bone healing area by F/U period were also collected for comparing UBE and microscopic discectomy. Visual analogue scale (VAS) scores, MacNab criteria, and restenosis or revision for evaluating disability and pain response were collected.
4. Measurement MethodWe measured the surface area using open-source software ImageJ bundle with 64-bit JAVA 1.8.0_172.ImageJ 1.5e (National Institutes of Health, Bethesda, MD, USA). First, we measured the area of interlaminar space in preoperative lumbar radiograph and then the area in postoperative lumbar radiograph. The laminectomy area is defined as the difference in area at postoperative and preoperative lumbar radiograph. The resulting red masks represent the laminectomy area (Figure 2A–C).
Laminectomy area=measured area at immediate postoperative–preoperative x-ray
Bone healing area is measured in the same manner after laminectomy. We measured the laminectomy area in lumbar radiograph at postoperative specific time and 6 months later than a postoperative specific time. The bone healing area is defined as the difference the area measured at postoperative specific time and 6 months later than a postoperative specific time. The interval period is 6 months, because the postoperative lumbar radiographs were performed every 6 months. For example, the bone healing area between POD 6 months and POD 12 months is defined as the difference in the measured area at POD 6 months and the measured area at POD 12 months (Figure 2D–F).
Bone healing=measured at a (α) – measured at a (α + 6 months), α=postoperative specific time
Alterations at the laminectomy site occur with bone healing. A 64-year-old woman presented with severe left leg radiating pain and left ankle dorsiflexion weakness (motor power: grade I). UBE discectomy with a left paraspinal approach was performed to decompress and dissect the compressive lesion. A lumbar radiograph was obtained in the outpatient clinic every 6 months. Imaging tests were performed until 30 months after UBE discectomy and show gradual healing of laminectomy, marked with a yellow outline in Figure 3. The remaining laminectomy area is defined as the difference in area measured preoperatively and at last F/U x-rays. For example, in Figure 3, there is a difference in area in the samples measured at Figure 3G and Figure 3A. In addition, bone recovery ratio is defined as the ratio of bone healing area to laminectomy area. For example, in Figure 3, bone recovery ratio is the difference in area measured at Figure 3B and Figure 3G divided into the difference in the area measured at Figure 3B and Figure 3A.
5. Statistical AnalysisStatistical analysis was performed using IBM SPSS Statistics ver. 25.0 (IBM Co., Armonk, NY, USA). All patients were matched by sex, age, BMI, BMD, and F/U duration and were divided into the “UBE group” and the “Microscopic group” by a 1:2 matching ratio. Continuous variables were expressed as mean and standard deviation. Significant differences in preoperative and postoperative VAS scores were determined using the paired t-test. Independent t-test was used to compare perioperative results, factors related with laminectomy area, and clinical results among the 2 independent groups. Mann-Whitney U-test or Wilcoxon signed-rank test was also used for nonparametric statistical analysis. A p-value of <0.05 was considered to indicate statistical significance.
RESULTSWe enrolled 22 patients who underwent UBE discectomy and 44 patients who underwent microscopic discectomy were suitable for our study. The patient demographics and perioperative results are summarized in Table 1. “Yes” indicates current user in each category in Table 1. For example, “Yes” in smoking is current smoker and “No” in smoking is ex- or nonsmoker. Sex, smoking, age, BMI, BMD, F/U duration, and operation level were not statistically different between the UBE and microscopic groups. The mean EBL was significantly less in the UBE group than in the microscopic group (48.18±61.36 mL vs. 110.45±177.45 mL, p=0.041). The mean hospital length of stay (LOS) was significantly shorter in the UBE group than in the microscopic group (6.14±1.88 days vs. 7.93±3.01 days, p=0.013).
The measurement of laminectomy area and clinical results are shown in Table 2. Laminectomy area in the UBE group was less than that in the microscopic group, but there was no statistical difference (83.50±53.22 mm2 vs. 98.40±72.45 mm2, p=0.396). The difference between laminectomy area and healing area is the bone defect remaining in the lamina that underwent surgery. In other words, the defect is the remaining laminectomy area after remodeling and the area that has not been healed. The remaining laminectomy area in the UBE group was significantly less than that in the microscopic group (13.91±25.16 mm2 vs. 53.84±55.19 mm2, p<0.001). Bone healing area and bone recovery ratio in the UBE group were significantly larger than those in the microscopic group (69.59±46.32 mm2 vs. 44.56±38.04 mm2, p=0.022; 85.42%±24.52% vs. 51.33%±28.19%, p<0.001, respectively) (Figure 4). We also measured the bone healing area at intervals of 6 months. Bone healing area did not differ by F/U period, but there was a higher trend of bone healing area by F/U period in the UBE group until 12 months after surgery, after which it was higher in the microscopic group, as shown in Figure 5.
The postoperative mean VAS scores improved significantly in both the UBE and microscopic groups (p<0.001) (Table 2). According to the Modified MacNab criteria, 13 patients (59.1%) in the UBE group and 23 patients (52.3%) in the microscopic group were rated as having excellent or good outcomes (Table 2). The mean VAS and the difference in preoperative and postoperative VAS scores of the UBE and microscopic groups were not significantly different. There was no case of restenosis or revision surgery due to bone remodeling.
DISCUSSIONMany previous studies have demonstrated that irrigation affects bone healing after osteotomy. In a previous study, patients undergoing osteotomy were divided into a group that maintained irrigation and a control group that did not. No significant difference in new bone formation was shown between the 2 groups. Pathologically, continuous irrigation during bone work resulted in increased new bone formation [25]. Therefore, it can be suggested that the difference in bone healing between the air-based microscopic group and the water-based UBE group was caused by the difference in thermal injury based on continuous irrigation.
Drilling parameters that can increase bone temperature and hence thermal osteogenesis include drilling speed, drill feed rate, drill diameter, drill point angle, drill material and wear, drilling depth, predrilling, drill geometry, cooling, and bone cortical thickness [26]. A strength of this study is that all surgeons performed laminectomy in UBE and microscopic groups using a match-head drill (9MH30, Medtronic/Midas Rex Legend Institute, Fort Worth, TX, USA). Depending on the center, there are institutions where laminectomy is performed using chisel without using drill. When laminectomy is performed using chisel, the thermal injury is not clear. However, in this study, it was possible to compare water-based and air-based surgery because only drill was used when conducting laminectomy. In addition, when using a drill during microscopic surgery, the amount of irrigation enough to remove only bone dust was minimal.
However, the other conditions were not constant. Also, since retrospective analysis was performed on actual patients, the laminectomy area might differ according to location of the lesion, such as extraforaminal lesion, up- and downward migration.
In addition, factors such as vitamin D, parathyroid hormone, and calcitonin level that can affect bone formation were not analyzed (patients receiving insulin and thiazolindinione were checked, but the number of patients was too small to be included: Thiazolindinione use in 1 UBE patient and 0 microscopic patients) [27]. Also, physical activity and caffeine were not considered [28-31]. However, drugs related with bone remodeling, such as proton pump inhibitor, nonsteroidal anti-inflammatory drugs (NSAIDs), antiepileptic drug, nicotine, and steroids, could be investigated [32-35], and pre- and postoperative use of NSAIDs and steroids was significantly different in UBE and microscopic groups (Table 3). For this reason, it can be inferred that the results of bone remodeling involve factors other than the difference between air and water environments.
The appropriate size of laminectomy for sufficient decompression can vary by lesion level. For example, since the interlaminar space of the L5/S1 level is generally wider than that of the L2/3 level, the minimum area required at the lower level might be smaller than that needed at the upper level. In addition, interlaminar space changes in different body positions, such as flexion and extension [36]. However, in the present study, there was no significant difference between UBE and microscopic groups, allowing an appropriate comparison (p=0.400) (Table 1).
A limitation of this study is use of lumbar radiograph rather than a 3-dimensional (3D) computed tomography (CT) reconstruction image to measure laminectomy area. Since the angle of the lamina is oblique rather than vertical, errors can occur when measuring laminectomy area by person and by surgical level. Such an error can result in over- or underestimation of laminectomy area. These may cause invalid and unreliable measurement. In addition, measurement by non-blinded assessor may result in information bias. And, in this study, most surgery was performed at levels L4/5 or L5/S1 (Table 1). A previous study showed no significant difference in L3 and L4 lamina angles in patients with or without disease [37]. However, no study has compared lamina angle at L4/5 and L5/S1 levels. To improve the accuracy of this study, it was necessary to perform CT tests on outpatients. However, performing CT tests for study can result in patients being exposed to unnecessary radiation. This could lead to ethical problems as well as medical problems, so it was not right to perform CT tests.
Another limitation is that the many internal organs around the lumbar spine can cause disturbances, such as by movement of bowel gas or organ movement. Such disturbances result in limitations in measuring the laminectomy area. To overcome this limitation, CT with 3D reconstruction can be used. However, as CT involves increased radiation exposure to the patient, it could not be performed in the present study (Figure 6). In addition, bone remodeling and quality cannot be evaluated simply by measuring the laminectomy area visible on lumbar radiograph but should be evaluated by various methods (mechanical testing, tissue pathology, dual energy x-ray, quantitative ultrasound, mineral/protein composition, etc.) [38].
Finally, the more bone healing after surgery, the more restenosis can be caused. However, in the results of this study, there was no difference in clinical outcomes between the UBE and microscopic group, and it did not cause restenosis in the UBE group. Despite these various limitations, this is the first comparative study about bone healing between air-based microscopic and water-based UBE discectomy.
CONCLUSIONBoth UBE and microscopic lumbar discectomy techniques are effective for treating patients with lumbar disc herniation. However, compared with the microscopic group, the UBE group had greater bone healing and recovery ratio, lower remaining laminectomy area and advantages in hospital LOS, and EBL. In addition, primary laminectomy area, bone healing area by F/U period, and clinical outcomes were not significantly different between the 2 groups. Thus, UBE discectomy is more likely to preserve the normal structure than is microscopic lumbar discectomy.
Figure 1.Inclusion flowchart for patients undergoing partial hemilaminectomy (UBE and microscopic surgery). POD, postoperative day; F/U, follow-up; BMI, body mass index; BMD, bone mineral density; UBE, unilateral biportal endoscopic. 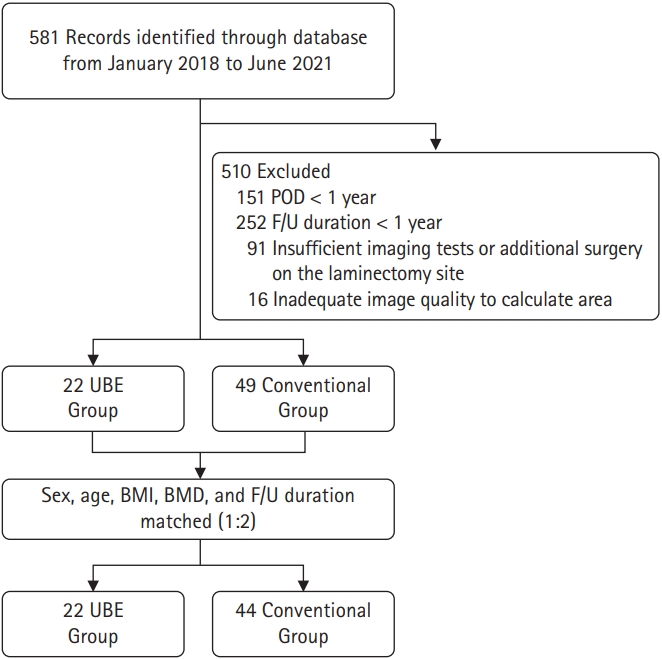
Figure 2.Laminectomy and bone healing areas. A preoperative lumbar radiograph in the standing position (A) and an immediate postoperative radiograph (B). (C) Measurement of the laminectomy area (red area). Laminectomy area=the area measured immediately postoperatively (B) – the area measured on the preoperative x-ray examination (A). The lumbar radiographs at the 6-month follow-up (D) and at the 12-month follow-up (E). (F) Measurement of the healing area (blue area). Healing area at 6–12 months postoperatively=the area measured at the 12-month follow-up (D) – the area measured at the 6-month follow-up (E). The healing area depended on the 2 periods of measurement. Interlamina area + laminectromy area (yellow area). 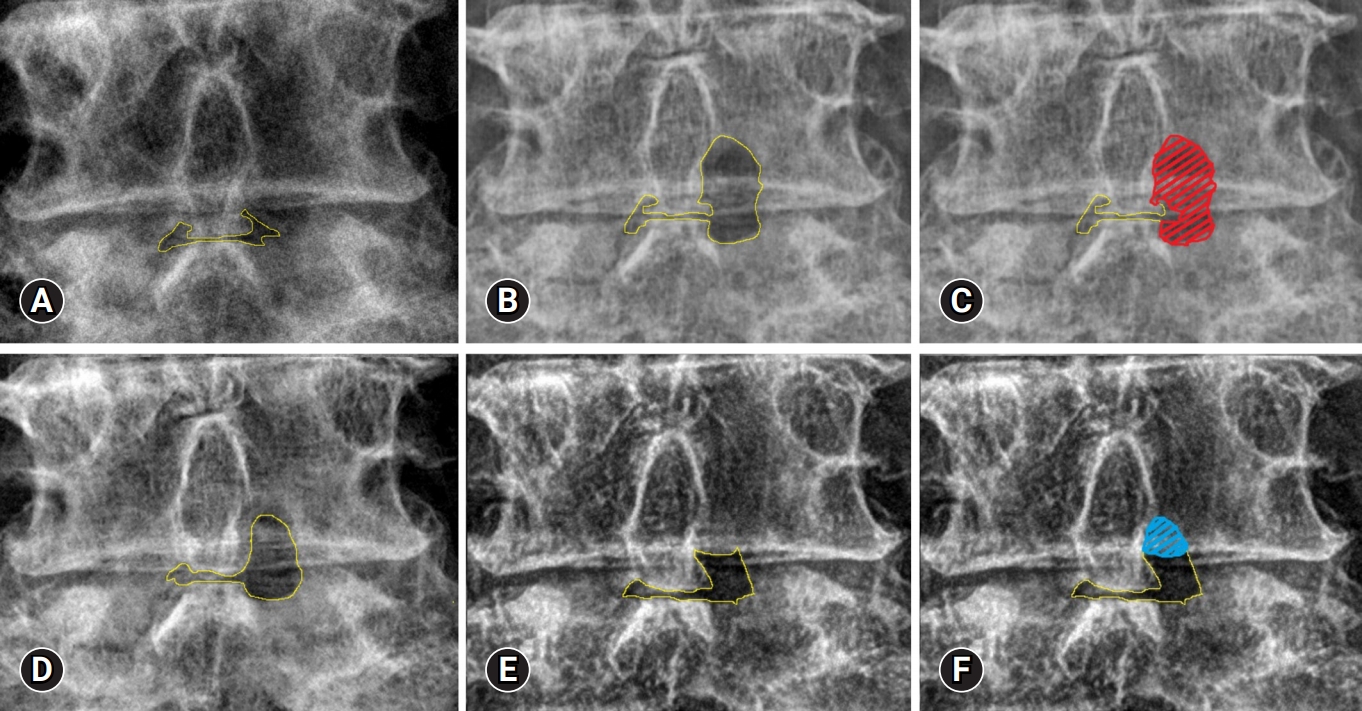
Figure 3.Serial changes in the laminectomy area on lumbar anteroposterior radiographs. Preoperative (A), immediately postoperative (B), postoperative 6-month (C), postoperative 12-month (D), postoperative 18-month (E), postoperative 24-month (F), and postoperative 30-month (G) images. Interlamina area + remaining laminectomy area (yellow area). 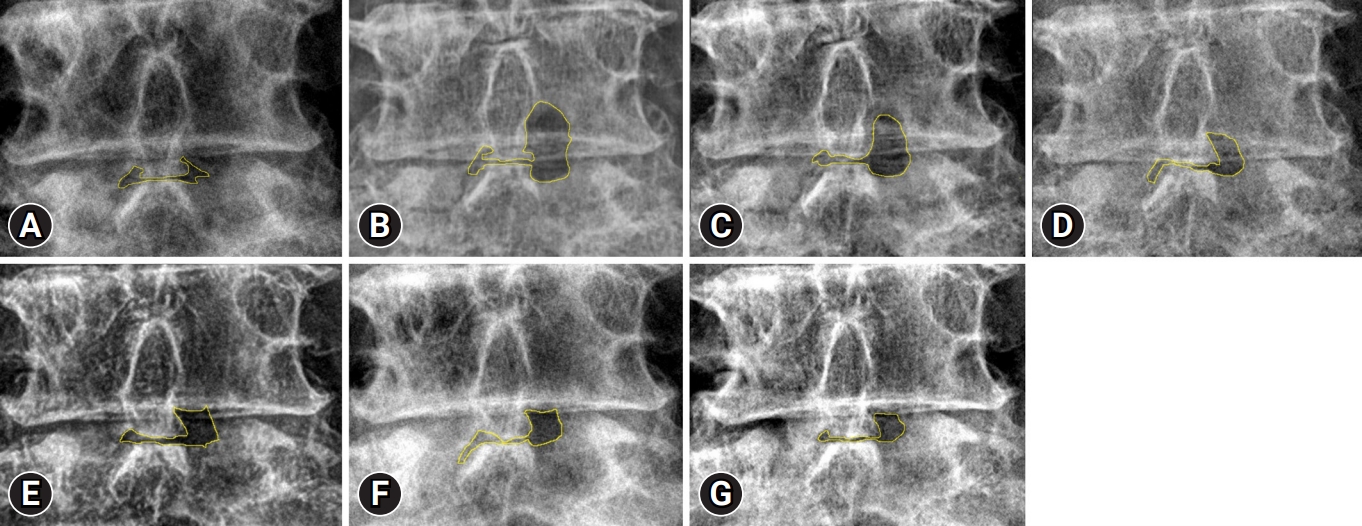
Figure 4.Comparison of laminectomy area, remaining laminectomy area, bone healing, and bone recovery ratio in unilateral biportal endoscopic (UBE) and microscopic groups. 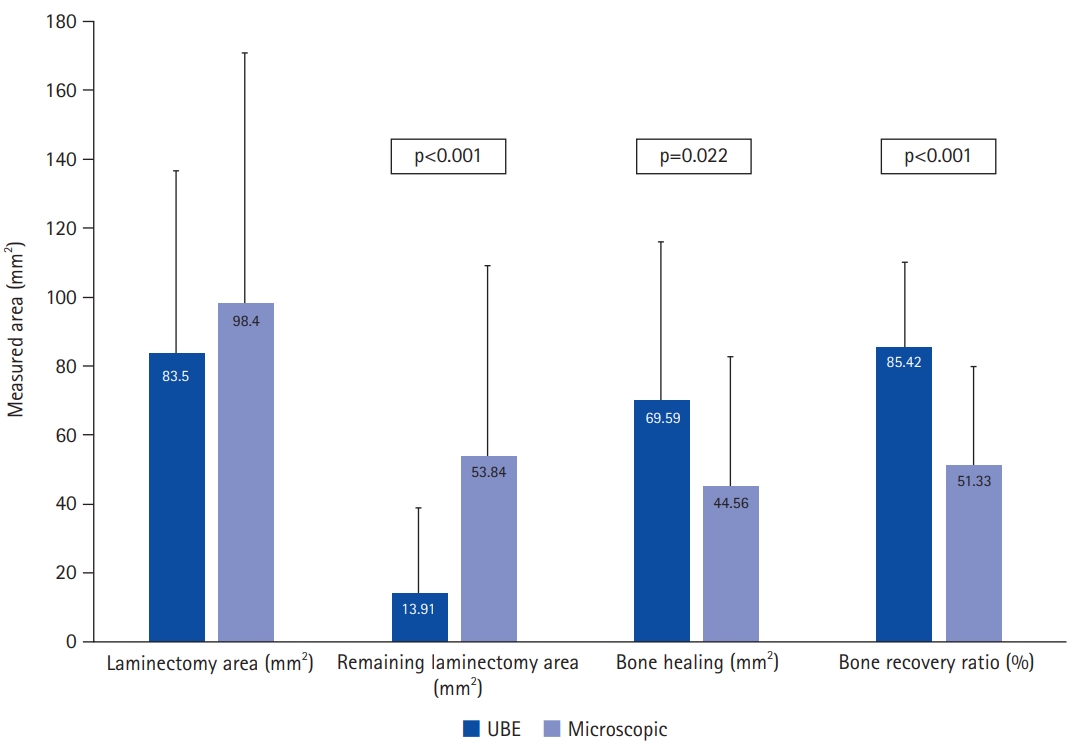
Figure 5.Trends in the bone healing rate in the unilateral biportal endoscopic (UBE) and microscopic groups. 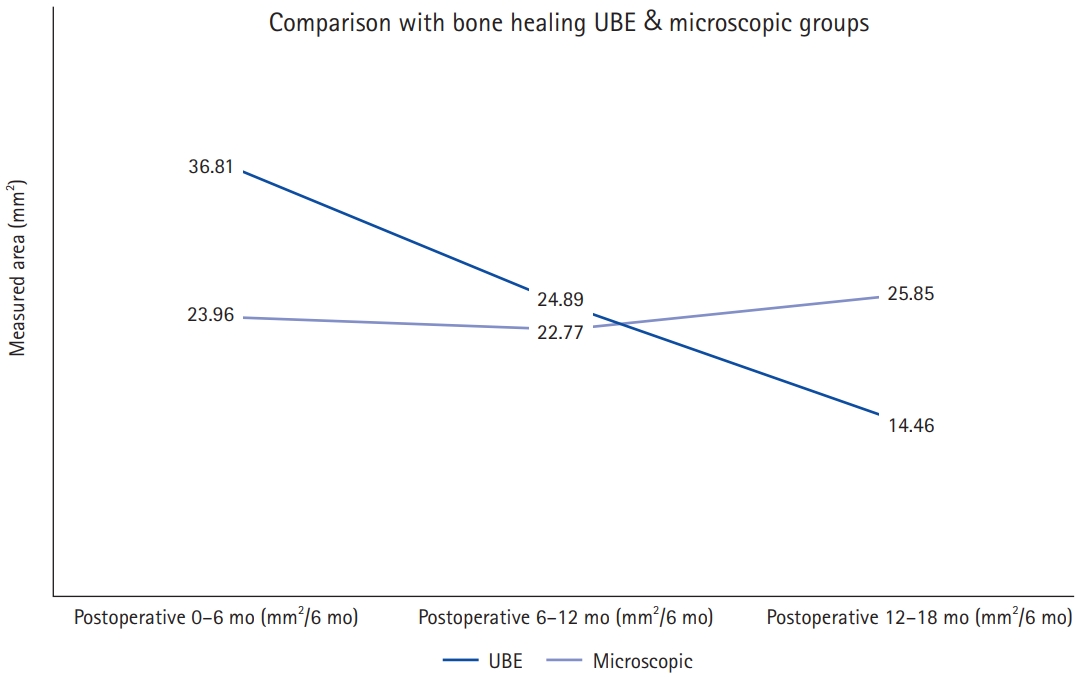
Figure 6.Anteroposterior view of a lumbar radiograph (A) and 3-dimensional spine computed tomography reconstruction (B). 
Table 1.Patient demographics and perioperative results Values are presented as number (%) or mean±standard deviation. UBE, unilateral biportal endoscopic; BMI, body mass index; BMD, bone mineral density; EBL, estimated blood loss; F/U, follow-up; LOS, length of stay. *p<0.05, statistically significant differences. †Independent t-test. ‡Mann-Whitney U-test were used for statistical analysis. Table 2.Laminectomy area changes and clinical results Values are presented as mean±standard deviation or number (%). UBE, unilateral biportal endoscopic; VAS, visual analogue scale; Δ VAS=preoperative VAS – postoperative VAS. The Mann-Whitney U-test and paired t-test were used. *p<0.05 statistically significant differences. †Laminectomy area minus bone healing area. ‡The ratio of bone healing area to laminectomy area. §Independent t-test. **p<0.001 versus preoperative data. Table 3.Factors associated with bone healing REFERENCES1. Robinson JS. Sciatica and the lumbar disk syndrome: a historic perspective. South Med J 1983;76:232–8.
2. Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis. Cochrane Database Syst Rev 2005;2005:CD001352.
3. Fox MW, Onofrio BM, Hanssen AD. Clinical outcomes and radiological instability following decompressive lumbar laminectomy for degenerative spinal stenosis: a comparison of patients undergoing concomitant arthrodesis versus decompression alone. J Neurosurg 1996;85:793–802.
4. Çelik SE, Çelik S, Göksu K, Kara A, Ince I. Microdecompressive laminatomy with a 5-year follow-up period for severe lumbar spinal stenosis. Clin Spine Surg 2010;23:229–35.
5. Mikami Y, Nagae M, Ikeda T, Tonomura H, Fujiwara H, Kubo T. Tubular surgery with the assistance of endoscopic surgery via midline approach for lumbar spinal canal stenosis: a technical note. Eur Spine J 2013;22:2105–12.
6. Thomé C, Zevgaridis D, Leheta O, Bäzner H, Pöckler-Schöniger C, Wöhrle J, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine 2005;3:129–41.
7. Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J 2009;18:672–8.
8. Ahn JS, Lee HJ, Choi DJ, Lee KY, Hwang SJ. Extraforaminal approach of biportal endoscopic spinal surgery: a new endoscopic technique for transforaminal decompression and discectomy. J Neurosurg Spine 2018;28:492–8.
9. Choi DJ, Kim JE, Jung JT, Kim YS, Jang HJ, Yoo B, et al. Biportal endoscopic spine surgery for various foraminal lesions at the lumbosacral lesion. Asian Spine J 2018;12:569–73.
10. Eum JH, Heo DH, Son SK, Park CK. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine 2016;24:602–7.
11. Kim JE, Choi DJ. Unilateral biportal endoscopic decompression by 30° endoscopy in lumbar spinal stenosis: technical note and preliminary report. J Orthop 2018;15:366–71.
12. Kim JE, Choi DJ. Bi-portal Arthroscopic Spinal Surgery (BASS) with 30° arthroscopy for far lateral approach of L5-S1 - Technical note. J Orthop 2018;15:354–8.
13. Kim JE, Choi DJ. Biportal endoscopic transforaminal lumbar interbody fusion with arthroscopy. Clin Orthop Surg 2018;10:248–52.
14. Soliman HM. Irrigation endoscopic decompressive laminotomy. A new endoscopic approach for spinal stenosis decompression. Spine J 2015;15:2282–9.
15. Torudom Y, Dilokhuttakarn T. Two portal percutaneous endoscopic decompression for lumbar spinal stenosis: preliminary study. Asian Spine J 2016;10:335–42.
16. Ahn Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices 2014;11:605–16.
17. Nellensteijn J, Ostelo R, Bartels R, Peul W, van Royen B, van Tulder M. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: a systematic review of the literature. Eur Spine J 2010;19:181–204.
18. Heo DH, Sharma S, Park CK. Endoscopic treatment of extraforaminal entrapment of L5 nerve root (far out syndrome) by unilateral biportal endoscopic approach: technical report and preliminary clinical results. Neurospine 2019;16:130–7.
19. Kim KR, Park JY. The technical feasibility of unilateral biportal endoscopic decompression for the unpredicted complication following minimally invasive transforaminal lumbar interbody fusion: case report. Neurospine 2020;17(Suppl 1):S154–9.
20. Song KS, Lee CW. The biportal endoscopic posterior cervical inclinatory foraminotomy for cervical radiculopathy: technical report and preliminary results. Neurospine 2020;17(Suppl 1):S145–53.
21. Song KS, Lee CW, Moon JG. Biportal endoscopic spinal surgery for bilateral lumbar foraminal decompression by switching surgeon's position and primary 2 portals: a report of 2 cases with technical note. Neurospine 2019;16:138–47.
22. Park MK, Son SK, Park WW, Choi SH, Jung DY, Kim DH. Unilateral biportal endoscopy for decompression of extraforaminal stenosis at the lumbosacral junction: surgical techniques and clinical outcomes. Neurospine 2021;18:871–9.
23. Epstein NE. Different surgical approaches to far lateral lumbar disc herniations. J Spinal Disord 1995;8:383–94.
24. Wiltse LL. The paraspinal sacrospinalis-splitting approach to the lumbar spine. Clin Orthop Relat Res 1973;(91):48–57.
25. Isler SC, Cansiz E, Tanyel C, Soluk M, Selvi F, Cebi Z. The effect of irrigation temperature on bone healing. Int J Med Sci 2011;8:704–8.
26. Augustin G, Zigman T, Davila S, Udilljak T, Staroveski T, Brezak D, et al. Cortical bone drilling and thermal osteonecrosis. Clin Biomech (Bristol, Avon) 2012;27:313–25.
27. Robinson ST, Shyu PT, Guo XE. Mechanical loading and parathyroid hormone effects and synergism in bone vary by site and modeling/remodeling regime. Bone 2021;153:116171.
28. Choi J, Choi SY, Lee SY, Lee JY, Kim HS, Lee SY, et al. Caffeine enhances osteoclast differentiation and maturation through p38 MAP kinase/Mitf and DC-STAMP/CtsK and TRAP pathway. Cell Signal 2013;25:1222–7.
29. Yuan Y, Chen X, Zhang L, Wu J, Guo J, Zou D, et al. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog Biophys Mol Biol 2016;122:122–30.
30. Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S. RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. Biomed Res Int 2020;2020:6910312.
31. Qi Z, Liu W, Lu J. The mechanisms underlying the beneficial effects of exercise on bone remodeling: roles of bone-derived cytokines and microRNAs. Prog Biophys Mol Biol 2016;122:131.
32. McCorry DJ. Effect of antiepileptic drugs on bone density in ambulatory patients. Neurology 2004;62:342; author reply 342.
33. Turan V, Mizrak S, Yurekli B, Yilmaz C, Ercan G. The effect of long-term nicotine exposure on bone mineral density and oxidative stress in female Swiss Albino rats. Arch Gynecol Obstet 2013;287:281–7.
34. Panday K, Gona A, Humphrey MB. Medication-induced osteoporosis: screening and treatment strategies. Ther Adv Musculoskelet Dis 2014;6:185–202.
35. Corrado A, Rotondo C, Mele A, Cici D, Maruotti N, Sanpaolo E, et al. Influence of glucocorticoid treatment on trabecular bone score and bone remodeling regulators in early rheumatoid arthritis. Arthritis Res Ther 2021;23:180.
36. Fu M, Li Q, Xu Y, Jiang T, Xiong M, Xiao J, et al. Variation in spatial distance between the lumbar interlaminar window and intervertebral disc space during flexion-extension. Surg Radiol Anat 2021;43:1537–44.
|
|
|||||||||||||||||||||||||||||||||||||