Comparative Analysis of Uniportal and Biportal Endoscopic Transforaminal Lumbar Interbody Fusion in Early Learning Stage: Technical Considerations and Radiological Outcomes
Article information
Abstract
Objective
This study compared the clinical and radiological outcomes of uniportal endoscopic and biportal endoscopic transforaminal lumbar interbody fusion (TLIF) for lumbar degenerative disease during the early learning stage of the technique.
Methods
We retrospectively analyzed patients who underwent uniportal endoscopic TLIF (n=15) and biportal endoscopic TLIF (n=19) between January and October 2021 during the first year of adoption of these techniques. Radiological parameters, including Bridewell fusion and subsidence grading, were evaluated by x-ray and computed tomography (CT) at 3-month, 6-month, and 1-year follow-up visits. Clinical outcomes were evaluated using the visual analogue scale (VAS) and the Oswestry Disability Index (ODI).
Results
Uniportal endoscopic TLIF showed significantly higher frequencies of intraoperative endplate injuries (uniportal [20%] vs. biportal [0%], p=0.01) and 1-year cage subsidence (uniportal [60%] vs. biportal [26.3%], p=0.04) than biportal endoscopic TLIF. The 1-year fusion rates did not differ significantly between the 2 surgical groups (uniportal [93.3%] vs. biportal [89.5%], p=0.37). Neural complications such as postoperative dysesthesia and dural tears occurred in uniportal endoscopic TLIF. There were no significant differences in the VAS for back and leg pain or ODI.
Conclusion
Complete endplate preparation under endoscopic guidance improved interbody fusion, and this procedure may be feasible in the early learning stage, regardless of the type of endoscope. Both endoscopic TLIF techniques achieved good clinical outcomes and fusion rates. However, unskilled use of the cage guide device caused endplate breakage and neural injury during uniportal endoscopic cage insertion. Uniportal endoscopic TLIF may require more experience for appropriate cage insertion.
INTRODUCTION
Minimally invasive surgery (MIS) lumbar interbody fusion, including microendoscopic and endoscopic approaches, minimizes injury to the normal lumbar structures and allows rapid recovery after surgery [1,2]. Two types of water-based endoscopic lumbar interbody fusion have been described: uniportal and biportal endoscopic systems. Both types of endoscopic transforaminal lumbar interbody fusion (TLIF) surgeries have developed rapidly using the posterolateral approach, which exploits the space created by unilateral facetectomy and laminotomy. Biportal endoscopic TLIF allows surgeons to use independent working portals for various cage insertions, including large oblique lateral interbody fusion and dual cages [3,4]. Furthermore, a novel cage guide device enables uniportal endoscopic TLIF to use large or multiple cages [5,6]. As a result of these technical developments, endoscopic TLIF surgeries have achieved good clinical outcomes and fusion rates comparable to those of conventional TLIF [3-5,7-9].
Previous studies showed good surgical outcomes by combining early and late experience stages. The good outcomes of the experienced stage may mask the less favorable results of the inexperienced stages. Uniportal and biportal endoscopic surgeries have different learning curves and use different endoscopic systems, instruments, and procedures for cage insertion. Technical differences can influence surgical outcomes, especially in the inexperienced early learning stage. Therefore, we compared the surgical techniques and their outcomes between uniportal and biportal endoscopic TLIF during the first year of starting each procedure.
MATERIALS AND METHODS
1. Study Patients
This retrospective analysis included patients who underwent uniportal or biportal endoscopic TLIF to treat lumbar degenerative disease between January 2021 and December 2021, the first year of the adoption of the 2 endoscopic techniques. Two experienced spine surgeons at a single center performed all procedures. A surgeon with 3 years of experience in uniportal endoscopic spine surgery learned and performed uniportal TLIF for consecutive patients (Figure 1A–D). Another surgeon with 2 years of experience in biportal endoscopic spine surgery started endoscopic TLIF (Figure 1E–H). We used a straight bullet-type 3-dimensional (3D)-printed titanium cage based on the disc space size (GS Medical, Cheongwon, Korea). The 3D cage was produced using a selective laser melting 3D printer (DMP 350, 3D Systems, Inc. Rock Hill, SC, USA), with a mean pore size of 500–1,100 µm and a mean porosity of 70%–80% (Figure 1C). We included all consecutive patients who met the following criteria: (1) persistent low back pain and radiating pain in the lower extremities despite 3 months of conservative treatment; (2) single-level endoscopic TLIF was performed because of spinal stenosis (central or foraminal), spondylolisthesis (degenerative or spondylolytic), segmental instability, or recurrent disc herniation; and (3) the duration of follow-up was more than 1 year.
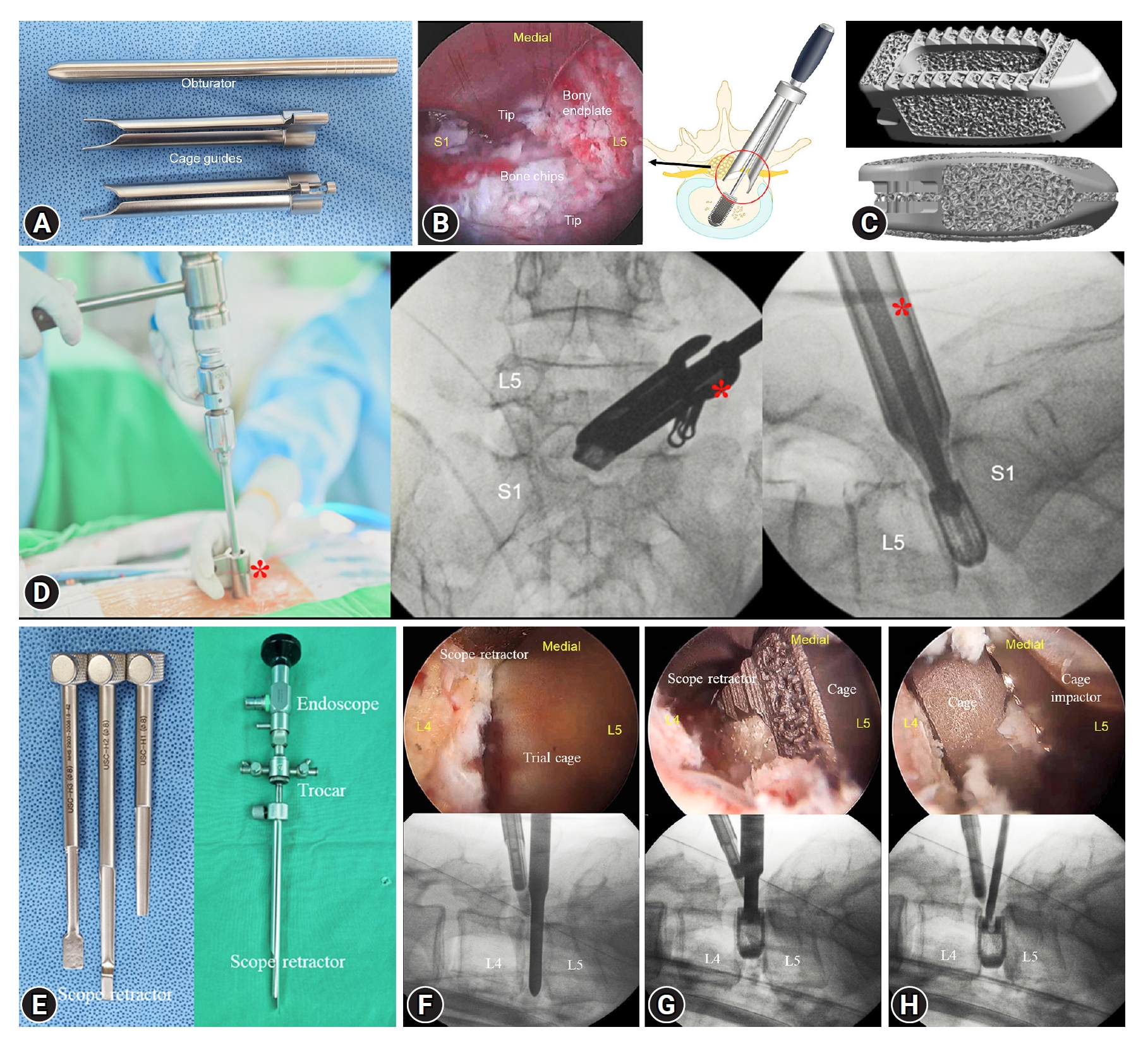
Instruments and surgical steps of uniportal and biportal endoscopic transforaminal lumbar interbody fusion (TLIF). (A) Customized cage guides for uniportal endoscopic TLIF. The cage guide has tailored bilateral tips for neural protection. (B) Bilateral distal ends are installed in the intervertebral disc space and protect the traversing and exiting nerve roots during cage insertion. (C) Three-dimensional-printed titanium interbody cage for TLIF. (D) Images of cage insertion include an intraoperative photograph (left) and C-arm images (middle and right). The cage guide (red asterisks) should be held to avoid retraction during cage insertion. (E) Scope retractors are installed on the endoscopic trocar during biportal endoscopic TLIF. (F) Trial cage insertion to determine the cage size. (G) Cage insertion. (H) Repositioning of the cage. All cage insertion steps are performed under endoscopic vision and C-arm guidance. The scope retractor protects the dural sac from the passing cage.
We excluded patients if they met any of the following criteria: (1) operation history of fusion at adjacent spinal levels; and (2) other lumbar spinal surgeries (discectomy or decompression) were performed simultaneously at different levels.
2. Surgical Procedures
1) Uniportal endoscopic TLIF
A uniportal endoscopic system was used, consisting of a 15° viewing angle, 10-mm outer diameter, 6-mm working channel, 125-mm working length, and 13.7-mm outer diameter working cannula (iLESSYS Delta, Joimax, Germany). Customized cage guides (MD & Co., Seoul, Korea) and impactors were used for cage insertion and repositioning (Figure 1A). The patient was positioned prone on the Wilson frame under general or epidural anesthesia. A 2-cm skin incision was made on the lateral border of the cephalad vertebral pedicle at the target level. After serial dilation, a working cannula was inserted and docked on the medial border of the inferior articular process. Ipsilateral total facetectomy and laminotomy were performed using endoscopic drills, punches, and osteotomes. The harvested bone was used as the bone fusion material. The ipsilateral ligamentum flavum of the spinal canal and foramen was completely removed for ipsilateral neural decompression. Contralateral decompression was performed in cases with bilateral symptomatic canal or foraminal stenosis. Then, annulotomy was performed using the endoscopic knife and punches. The cartilaginous endplate was peeled off from the bony endplate using the curved dissectors and removed using the forceps. We did not use the curettes or shavers during endplate preparation due to the risk of bony endplate injury. All the discectomy and endplate preparation procedures were performed under direct endoscopic vision while protecting the neural structures using the working cannula.
Subsequent procedures were performed under C-arm guidance after endoscopy removal. An obturator was inserted into the disc space through the working channel and the working channel was then withdrawn. Finally, the cage guide was slid into the disc space along the obturator. Two separate tips of the cage guide were docked into the disc space, while simultaneously protecting the traversing and exiting nerve roots (Figure 1B). We inserted trial cages of serial size into the disc space to determine the cage size. Autogenous bone chips were inserted into the disc space (Figure 1B) by using a specialized funnel-shaped device. The cage was inserted obliquely deep into the disc space under C-arm guidance (Figure 1C, D). The endoscope accessed the cage, and the cage was repositioned to a more appropriate position using impactors. After hemostasis, the endoscope was removed. A closed drainage tube was inserted prior to screw fixation. A skin incision was made for endoscope entry and 3 additional skin incisions were made for percutaneous pedicle screw fixation.
2) Biportal endoscopic TLIF
We used biportal endoscopic systems (4 mm, 0° endoscope), a toolkit set, a customized scope retractor, and a working sheath (MD & Co.) [7,10,11]. The surgical preparation steps were similar to those of the uniportal endoscopy. Two ipsilateral skin incisions were made at the lateral border of the pedicles for 2 portals (endoscopic and working portals). After serial dilation, an endoscopic trocar and working sheath were inserted into each portal. The endoscope and instruments were introduced through the trocar and the working sheath to access the initial docking area (Figure 1E). Subsequently, facetectomy, laminotomy, flavectomy, and discectomy were performed in a manner similar to uniportal endoscopic TLIF. A scope retractor, installed on the trocar, was used for neural retraction during endplate preparation and cage insertion (Figure 1E). After the harvested bone chips were placed in the intervertebral disc space, the cage was obliquely inserted into the disc space under the guidance of an endoscopic view and a C-arm image (Figure 1F–H). Inappropriate cage access can be immediately detected by endoscopic view. The cage was repositioned to the appropriate location using cage impactors. Ipsilateral skin incisions of the 2 portals and 2 additional skin incisions were made for percutaneous fixation of the pedicle screws.
3. Clinical Data Collection
This study was approved by the Wiltse Memorial Hospital Ethics Committee (NR-IRB 2022-W12). Physicians collected clinical information preoperatively, postoperatively, and at the 3 and 6 months, and final follow-up in the ward and outpatient department. Patient characteristics including sex, age, duration of follow-up, nature of surgery, and complications were recorded. Visual analogue scale (VAS) scores for back and leg pain, the Oswestry Disability Index (ODI), and MacNab criteria for evaluating disability and pain responses were collected.
4. Radiological Data Collection
We performed radiography and computed tomography (CT) to assess the fusion and subsidence grades at 3 and 6 months, and 1 year. Bone fusion was assessed using the Bridwell fusion grading system based on CT scan images [12]. The Bridwell fusion grades are classified as follows: grade I, fused with trabecular bone formation; grade II, graft intact, not fully remodeled and incorporated, but no lucency present; grade III, graft intact, potential lucency present at the top and bottom of the graft; and grade IV, fusion absent with collapse/resorption of the graft (Figures 2A–D). The fusion rate was calculated using the sum of grades I and II. Subsidence was measured from standing neutral lateral radiographs with parallel endplate at index level (Figure 2E). The subsidence was then categorized: grade 0, 0%–24% loss of postoperative disc height; grade I, 25%–49% collapse; grade II, 50%–74% collapse; and grade III, 75%–100% collapse [13]. The endplate injury was evaluated on lying down lateral x-rays performed on postoperative day 1 (Figure 2F). It is defined as vertebral body collapse of >25% of the cage height through bony endplate breakage, which might have occurred during cage insertion.
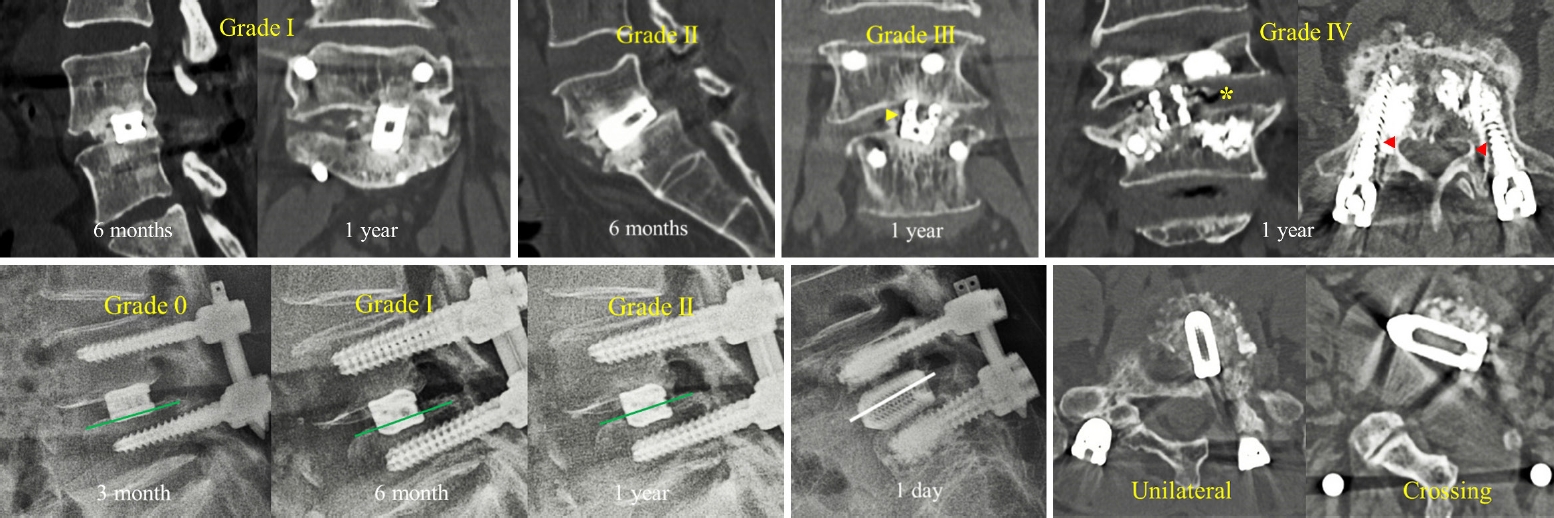
Assessments of radiological parameters, including fusion grading, subsidence grading, and cage positions. Fusion evaluation was performed using the Birdwell fusion grading system on the computed tomography (CT) scan. (A) Grade I at 6 months and 1 year. (B) Grade II at 6 months. (C) Grade III at 1 year; lucency is observed around the cage (yellow arrowhead). (D) Grade IV at the 1-year follow-up. Yellow asterisk: intradiscal vacuum after graft resorption. Red arrowheads: pedicle screw loosening is visible. Subsidence grading assessed using the lateral x-ray image. (E) Grade 0 at the 3-month follow-up (left), grade I at the 6-tmonth follow-up (middle), and grade II at the 1-year follow-up (right). (F) A lateral x-ray image shows the impaction of the cage in the vertebral body through the breakage of the endplate. We identified an endplate injury on the postoperative 1-day x-ray image. (G) The cage positions were assessed on the CT axial image.
The preoperative disc height of the index level was calculated by dividing the sum of the heights of the anterior, middle, and posterior intervertebral discs by three [14]. The height gap between the preoperative intervertebral disc and the inserted cages was analyzed. The cage position was assessed from the axial cut of the CT scan. If 25%–50% of the cage crossed the midline, it was classified as crossing, otherwise it was classified as unilateral (Figure 2G). Two experienced surgeons who did not perform endoscopic TLIF measured the radiological parameters, and the average value was used for statistical analysis.
5. Statistical Analysis
The Wilcoxon rank-sum test and Fisher exact test were used for statistical analysis using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). A p-value of <0.05 was considered significant.
RESULTS
We included 15 (8 men, 7 women) and 19 (7 men, 12 women) patients who underwent uniportal and biportal endoscopic TLIF, respectively. The mean age was 69.5±7.6 years in the uniportal endoscopy group and 60.4±7.2 years in the biportal endoscopy group (Table 1). The mean duration of the follow-up was 14.4±1.1 months and 13.7±1.4 months in the uniportal and biportal endoscopy group, respectively. The mean hospital stay in the uniportal endoscopy group was 12.3±3.3 days and 11.5±2.7 days in the biportal endoscopy group. The operation time was significantly longer in the biportal endoscopy group than in the uniportal endoscopy group (uniportal, 170.7±18.4 minutes; biportal, 204.7±47.9 minutes; p=0.02). Biportal endoscopic surgery showed a higher proportion of bilateral decompression (unilateral: bilateral, 13:2 in uniportal, 10:9 in biportal endoscopy) (Table 1). The most common fusion sites in both groups were level L4–5 (uniportal, 53%; biportal, 58%), and spondylolisthesis was the most common operating disease in the uniportal and biportal groups (53% and 47%, respectively). There were no significant differences in terms of bone marrow bone marrow density (Table 1).
The postoperative surgical site hematoma was found on postoperative magnetic resonance imaging; One case was uniportal endoscopy and 4 cases were in the biportal endoscopy cases. Patients experiencing hematoma resolved with conservative treatment (Table 1). Postoperative dysesthesia caused by nerve root retraction was observed in 4 patients who underwent uniportal endoscopic TLIF (Figure 3A). Symptoms of dysesthesia symptoms appeared 3 days after surgery and resolved with conservative treatment. During the uniportal endoscopic cage insertion, a one dural tear occurred at the ventrolateral border of the dural sac (Figure 3B, C). The dural defect was repaired by conversion to open microscopic surgery (Table 1).
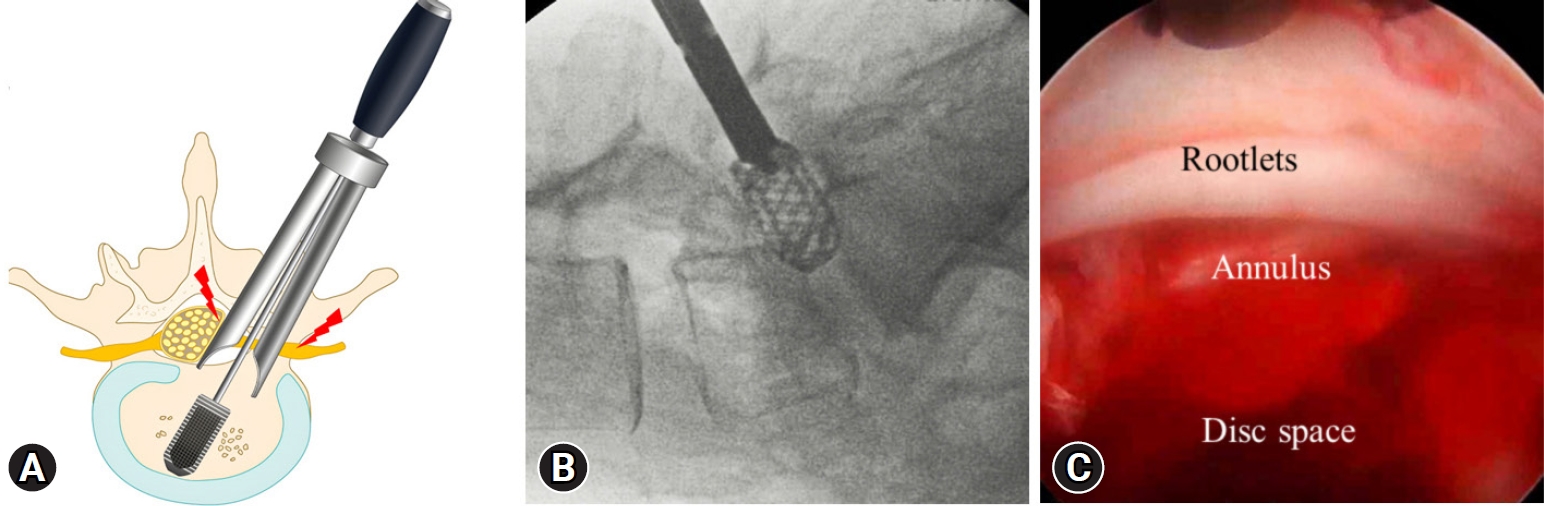
Neural complications. (A) Excess neural retractions can occur during dural cage insertion performed without endoscopic guidance. Nerve root retraction injuries (red lightning bolt marks) can occur traversing or exiting the nerve root or simultaneously. (B) In spondylolisthesis at the L5–S1 levels, proper use of the cage guide is complicated, and the cage guide is removed early for appropriate cage direction. However, this technique may cause a dural tear. (C) Intraoperative photograph of dural tear observed after cage insertion. A dural tear occurred on the ventrolateral side of the dura sac.
The successful fusion rate (grade I + II) according to the Bridwell fusion grading system was 80% in the uniportal and 84.2% in the biportal endoscopy group at the 6-month follow-up. At the 1-year follow-up, fusion rates of 87% and 89.5% were observed in the uniportal and biportal endoscopy groups, respectively (Table 1). The fusion rate did not show significant differences between the 2 groups at the 6-month or 1-year follow-up. A grade IV case was found in the uniportal endoscopy group at the 1-year follow-up (Table 1).
Three cases of endplate injury were observed on the postoperative 1-day lateral x-ray were observed in the uniportal endoscopy group (Figure 4). However, there were no endplate injuries in the biportal endoscopy group (Table 2). Total cage subsidence, including grades I, II, and III, was significantly higher in the uniportal endoscopy group than in the biportal endoscopy group at the 3-month follow-up (60% vs. 15.8%, p=0.01) (Table 2). Three cases of endplate injury in the uniportal endoscopy group progressed to grade II subsidence at the 3-month follow-up. The biportal endoscopy group did not show any evidence of severe subsidence (grade II or III) at the 3-month follow-up (Table 2). At the 1-year follow-up, the uniportal endoscopy group had significantly higher total cage subsidence than the uniportal endoscopy group (60% vs. 26.3%, p=0.04) (Table 2).
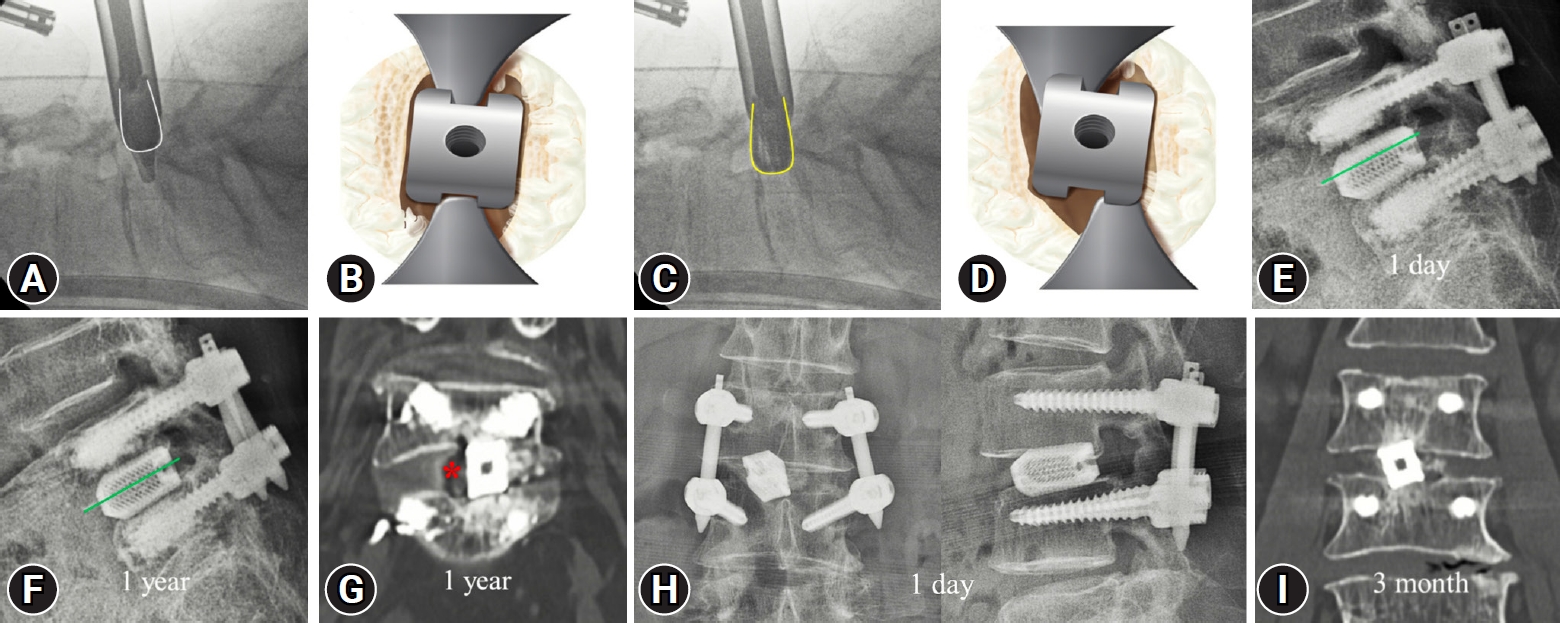
Cause of endplate injury and representative cases thereof. (A) Appropriate position of the cage guide. Distal tips are located in the disc space, and their direction is parallel to a cage (white line contour). (B) The illustration shows the appropriate position of the cage and distal tips of the guide. (C) The deep-inserted guide tips are stuck in the disc space and distort the cage direction (yellow line contour). (D) Distorted guide tips cause the cage to rotate. A rotating cage is inserted into the disc space by breaking the bony endplate. (E) An endplate injury was observed in the upper-level vertebral body 1 day after surgery. (F) Endplate injury progressed to grade II subsidence at the 1-year follow-up. (G) A 1-year computed tomography (CT) image shows graft resorption (red asterisk) and poor fusion state (grade III Bridewell grading system). (H) A rotated cage was observed on postoperative day-1 x-ray images. (I) A 3-month CT image showing the grade 1 subsidence with rotated cage position.
The height of the preoperative disc did not differ significantly between the 2 groups. The uniportal endoscopy group used significantly higher cages than the biportal endoscopy group (uniportal, 11.9±0.9 mm vs. biportal, 10.7±1.1 mm; p<0.001) (Table 2). However, the height gap between the inserted cages and the height of the preoperative disc did not reveal significant differences (uniportal, 6.3±1.9 mm vs. biportal, 4.9±2.4 mm; p=0.07) (Table 2). The cage position was 60% unilateral and 40% crossed in the uniportal endoscopy group; and all cases in the biportal endoscopy group showed the crossed cage position.
VAS scores for back and leg pain and ODI improved markedly at the final follow-up, and there were no significant differences between the 2 groups (Table 3). The MacNab criteria revealed successful clinical outcomes in both groups (Table 3). One patient with pseudarthrosis in the uniportal endoscopy group showed a fair response due to low back pain recurrence.
DISCUSSION
Trans-Kambin uniportal endoscopic lumbar interbody fusion was performed using the Kambin triangle, which is used for transforaminal endoscopic lumbar discectomy. Although the Kambin triangle expands by partial superior articular process resection, the space is limited by the exiting nerve root [15,16]. Therefore, the insertion of the large cage is restricted because of the narrow Kambin space and the possibility of exiting nerve root injury [17].
Uniportal endoscopic lumbar interbody fusion rapidly develops using the posterolateral approach similar to MIS TLIF. The space created by ipsilateral facetectomy and laminotomy offers a broad entry for large and variously shaped cage insertions. Kim et al. [9] introduced a novel cage guide device (Harrison cage glider) for safe delivery into the space of the intervertebral disc. The authors used bilateral distal tips that were inserted into the intervertebral disc space and simultaneously protected the traversing and exiting nerve roots during cage insertion. Due to guarantee of neural protection, larger angled cages and multiple cages could be used for uniportal endoscopic TLIF. These procedures achieved better clinical outcomes and fusion rates with less subsidence than microscopic MIS TLIF in the mid-term evaluation.
During uniportal endoscopic TLIF, the endoscopy and instruments use the same closed space inside the working cannula. A cage larger than the diameter of the working cannula blocked the endoscopic view. The cage must be inserted only under the C-arm guidance, without endoscopic observation. Therefore, the uniportal endoscopic TLIF should use a cage guide device for the insertion of a large cage, and skillful manipulation of the cage guide is essential to reduce complications. This study showed several complications associated with the unskilled use of cage guides during uniportal endoscopic TLIF.
In the early learning stage of uniportal endoscopic TLIF, the cage guide tips are deeply inserted into the intervertebral disc space due to the fear of producing dural tears. The deeply inserted guide tips often twisted and were stuck in the disc space, distorting the cage insertion path. Inappropriately accessed cages may be inserted into the disc space by breaking the bony endplate. In this study, 3 endplate injury cages were detected in the postoperative 1-day x-ray image, and progressed to grade II subsidence at the 3-month follow-up. One patient progressed to pseudoarthrosis at the 1-year follow-up and showed a poor response to the MacNab criteria.
Endplate injuries can be affected by poor bone quality or the use of cages with excessive height. We measured the T score and the height gap between the preoperative disc height and the inserted cage height. Comparison of the 2 parameters did not show a significant difference between the 2 surgical groups. Furthermore, biportal endoscopic TLIF did not cause endplate injury. As a result, technical factors may have influenced endplate injury. Biportal endoscopic surgery allows surgeons to use both hands by separating the endoscopic and working portals, thereby facilitating the clear visualization of cage insertion procedures. Biportal endoscopic TLIF can be performed without the help of a cage guide device; thus, smooth cage manipulation is possible when the appropriate position is used.
Different techniques can also affect the position of the cage in the disc space. During uniportal endoscopic TLIF, deeply inserted bilateral tips restrict the manipulation of the cage guide device. Therefore, insertion of the cage by crossing the midline is difficult and inserted cases are commonly placed in the ipsilateral space of the intervertebral disc. In this study, 60% of the patients who underwent uniportal endoscopic TLIF presented unilateral cage positions. Conversely, biportal endoscopic TLIF revealed a 100% crossing cage position, which may have been due to the flexibility of biportal endoscopic surgery.
It is impossible to assess whether neural retraction causes a potential dural tear while the cage passes through the annulus hole in uniportal endoscopic TLIF. Therefore, dural tears were confirmed endoscopically after cage insertion, and neural retraction injury was detected by postoperative neurological examinations. In this study, 3 postoperative dysesthesias were observed 3 days after surgery. Excess spreading of guide tips may induce nerve root retraction injury. It was difficult to determine the origin of dysesthetic pain, whether it was attributed to traversing or exiting nerve roots. A dural tear occurred on the ventrolateral side of the dural sac during cage insertion, which was confirmed by endoscopic vision after cage guide removal. The cause of the dural tear was unclear and may have occurred from the passing cage or from the rotating cage guide tip. We converted this to microscopic surgery and directly repaired the dural tear with a meticulous suture.
Neural complications, such as postoperative dysesthesia and dural tear, occurred in uniportal endoscopic TLIF; however, biportal endoscopic TLIF did not show neural injury. Biportal endoscopic TLIF simultaneously allows all cage insert steps under endoscopic vision and C-arm guidance and surgeons can frequently observe neural structures and appropriately manipulate them to avoid injury. Real-time endoscopic observation, such as biportal endoscopic TLIF, seems to be critical for preventing neural damage during cage insertion, especially in an inexperienced stage.
Despite differences in cage insertion techniques, the 2 surgical groups showed good fusion rates without a significant difference at the 6- and 12-month follow-ups. One-year fusion rates (uniportal, 87%; biportal, 89.5%) were similar to the previously reported results of endoscopic lumbar interbody fusion [4,18,19]. Ao et al. [20] reported an 85.3% fusion rate using CT (29 of 34 patients) 12 months after uniportal endoscopic trans-Kambin TLIF.
Endplate preparation is essential to enhance interbody fusion. Both endoscopic systems are inserted into the intervertebral disc space, and complete endplate preparation can be performed under a clearly magnified endoscopic view, even in the early learning stage. As a result, good fusion rates were obtained in the inexperienced state, similar to those obtained by experienced surgeons, which may be an advantage of endoscopic TLIF surgery.
The present study has several limitations that should be considered. This was a retrospective study with a small sample size. A prospective study with a larger sample size is required to confirm the results of this study. The observed disparity in mean ages between the study groups may have influenced the cage subsidence. Surgical techniques and skills may have significantly influenced the study results. A larger number of surgeons should be included in future multicenter studies to clarify the findings of this study.
CONCLUSION
Complete endplate preparation under endoscopic guidance enhanced interbody fusion, and this procedure may be feasible in the early learning stage, regardless of the type of endoscope used. Therefore, both uniportal and biportal endoscopic TLIF showed good clinical outcomes and fusion rates. However, unskilled use of the cage guide device resulted in endplate breakage and neural injury during uniportal endoscopic cage insertion. Uniportal endoscopic TLIF may require more experience for appropriate cage insertion and the reduction of neural complications.
Notes
Conflict of Interest
Ji Yeon Kim, Hyeun Sung Kim, and Dong Hwa Heo are the Editor-in-Chief and Editorial Board of the Journal of Minimally Invasive Spine Surgery and Technique and were not involved in the review process of this article.
All authors have no other potential conflicts of interest to declare relevant to this article.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.