Biportal Endoscopic Trans-Kambin Lumbar Interbody Fusion: Surgical Techniques and Treatment Outcomes
Article information
Abstract
Objective
Lumbar interbody fusion with cages is a commonly used surgical solution for spinal conditions that do not respond to conservative management. Biportal endoscopic trans-Kambin lumbar interbody fusion (BE-KLIF) is a modern technique that offers several benefits over prior techniques, including early ambulation, reduced postoperative pain, and shorter hospital stays.
Methods
This retrospective study enrolled 128 patients who underwent BE-KLIF between March 2018 and August 2022. The primary indications for surgery were segmental lumbar listhetic instability, failed decompression with neuroforamen stenosis, adjacent segment disease, and burst fracture of the lumbar vertebrae. The outcome measures included Oswestry Disability Index (ODI) and visual analogue scale (VAS) scores, and postoperative fusion status at 1 year after the procedure was graded using the Bridwell fusion grading scale.
Results
BE-KLIF yielded significant improvements in patient outcomes. Successful fusion was achieved in 91.8% of segments. The mean ODI score was significantly lower at the 1-year follow-up than before the procedure. Similarly, VAS scores for leg and back pain significantly improved after the procedure. Seven early and 3 late postoperative complications were observed. The mean length of hospital stay was shorter for BE-KLIF than for the older transforaminal lumbar interbody fusion technique.
Conclusion
BE-KLIF resulted in less bone removal, preservation of the facet joint, facilitation of a more oblique trajectory, and potential for larger cages with wider effacements compared with prior techniques. However, the technique lacks central and contralateral decompression. We recommend performing same-side unilateral laminotomy with bilateral decompression to provide central and contralateral decompression.
INTRODUCTION
Lumbar interbody fusion with cages has long been considered the final solution for lumbar segmental degenerative and listhetic end-point diseases that do not respond to conservative management or decompression procedures. Several approaches for performing lumbar interbody fusion—including anterior, lateral, oblique and posterior approaches have been proposed. The posterior approach initially gained popularity among spine surgeons due to its simplicity, ease of access for concomitant decompression and instrumentation, high margin of safety, and reliability. Over time, the conventional opened posterior transforaminal approach, known as transforaminal lumbar interbody fusion (TLIF), became less popular due to violation of spinal musculatures. A minimally invasive version of the TLIF (MIS-TLIF) approach was developed. This approach was made possible due to advancements in tubular retractors and microscopic instrumentation. These advancements have led to significant improvements in clinical outcomes. In recent years, the introduction of endoscopic techniques has revolutionized the field even further. These techniques, while achieving results similar to those achieved using conventional and minimally invasive approaches, further minimize the violation of soft tissues, thereby improving patient outcomes.
Initial attempts at uniportal endoscopic techniques for lumbar interbody fusion with cages, as described by Morgenstern et al. [1], were challenging and often resulted in neurapraxia. Furthermore, the instrumentation necessary for the techniques was expensive. These initially attempted techniques have since been refined, and the refined techniques have become popular. A trans-Kambin approach (KLIF) has been developed that is relatively simple and can be performed when the patient has received only local anesthesia, as demonstrated by Sairyo et al. [2].
In 2020, Heo proposed an endoscopic technique for lumbar transforaminal interbody fusion with cages that uses a biportal posterolateral approach (BE-TLIF). This technique has since been adopted and described in several articles [3-5]. The BE-TLIF approach was evolved from the MIS-TLIF postero-lateral approach but discarded the expandable tubular retractor and instead uses endoscopic video-guided irrigation system to sacrifice the facet joint, with a slightly oblique trajectory employed to insert one or 2 cages for interbody fusion. Several papers—including those by Heo et al. [3], Kim and Choi [4], Pao [6], and Kang et al. [7]—have been published on biportal endoscopic techniques and have demonstrated the significant improvements these techniques yield compared with prior MIS-TLIF techniques. The improvements yielded by BE-TLIF techniques include early ambulation, reduced postoperative pain, and a shorter hospital stay [8]. However, these techniques also have several limitations, such as potential injury to traversing and exiting nerve roots due to obliteration of the facet joint, limited trajectory options, and relatively small dimensional cage effacements.
Recently, a modified TLIF technique that involves extraforaminal lumbar interbody fusion was proposed. This technique focuses on preserving the facet joint by using a KLIF with a more oblique trajectory to insert a single larger interbody cage. The goal is to achieve better disc space effacement and reduce the risk of injury to traversing and exiting nerve roots. However, no large studies have investigated the effectiveness of this new technique.
This study retrospectively examined patients who underwent biportal endoscopic trans-Kambin lumbar interbody fusion (BE-KLIF). The author used instead with abbreviation of BE-KLIF which in line with uniportal KLIF techniques but with biportal approach.
MATERIALS AND METHODS
This retrospective study was conducted at the Yonghe Cardinal Tien Hospital, New Taipei City, Taiwan. The study was conducted with Declaration of Helsinki and was approved by the Institutional Review Board of Yonghe Cardinal Tien Hospital. Informed consent of publication was provided by each patient. In total, 128 participants who underwent BE-KLIF (involving 168 segments) between March 29, 2018, and August 30, 2022, were enrolled. The primary indication for the surgery was as follows:
(1) Segmental lumbar listhetic instability with neurologic symptoms refractory to conservative medical or physical therapies at our hospital or other clinics for more than 3 months.
(2) A history of failed decompression with severe neuroforamen stenosis with associated back and root syndrome and failed conservative medical or physical therapies.
(3) Adjacent segment disease after prior fusion surgery with cranial listhetic instability and neurologic symptoms.
(4) Bursting fracture of lumbar vertebrae with instability requiring cage fusion for 360° fusion with posterior instrumentation. One unique case, L5 tumor metastatic lesion with resultant bursting fracture required cage fusion for augmentation of stability after cemented posterior instrumentation.
All patients received a preoperative risk assessment brochure and gave written informed consent. All cages were polyetheretherketone cages 10 mm in width. The cages were obtained from Medtronic (Minneapolis, MN, USA) or Wiltrom (Hsinchu, Taiwan). The length of each cage was chosen based on the findings of an intraoperative evaluation under fluoroscopy.
After the fusion procedure, biframe or uniframe instruments were used to provide posterior stability. Patients who were aged <75 years and who had an active lifestyle, grade I or II spondylolisthesis, and a difference in listhetic distance of ≥3 mm received biframe instruments.
Patients who were aged ≥75 years or who had a sedentary lifestyle, no instability, or a limited listhetic distance (<3 mm) received uniframe instruments.
1. Evaluation Criteria for Clinical Data and Outcomes
All patients underwent a preoperative lumbar spine plain x-ray, lumbar dynamic view, computed tomography, or magnetic resonance imaging evaluation. The outcome measures were the preoperative and 1-year postoperative Oswestry Disability Index (ODI) and visual analogue scale (VAS) score for leg pain and back pain. Due to difficulties in assessing blood loss during the procedure, we selected a group of 37 patients who underwent single-level fusion and evaluated their preoperative and postoperative day 1 complete blood count data. Operating times were divided into 3 categories: cage (from incision to cage insertion), decompression (from the end of cage insertion to decompression of the nerve root), and instrumentation (from the end of decompression to finalizing the posterior instrumentation).
Postoperative fusion status was evaluated 1 year after the procedure by using serial radiography, including plain x-ray, dynamic view, and computed tomography. Fusion status was graded using the Bridwell [9] fusion grading scale, which has the following grades:
Grade I: fusion with remodeling and trabeculae.
Grade II: graft intact, not fully remodeled or incorporated but no lucency present.
Grade III: graft intact; potential lucency present at top and bottom of the graft.
Grade IV: fusion absent with collapse or resorption of the graft.
2. Surgical Techniques
1) Anesthesia, positioning, and surgical draping
All patients were given general anesthesia by endotracheal intubation and placed in a prone position on a radiolucent 4-point support frame that maintained the lumbar spine in lordotic curvature. Water-seal draping was applied with continuous saline irrigation to allow for free inflow and outflow of fluid to a 360° water bag without dam structure to provide radiolucent fluoroscopic guidance.
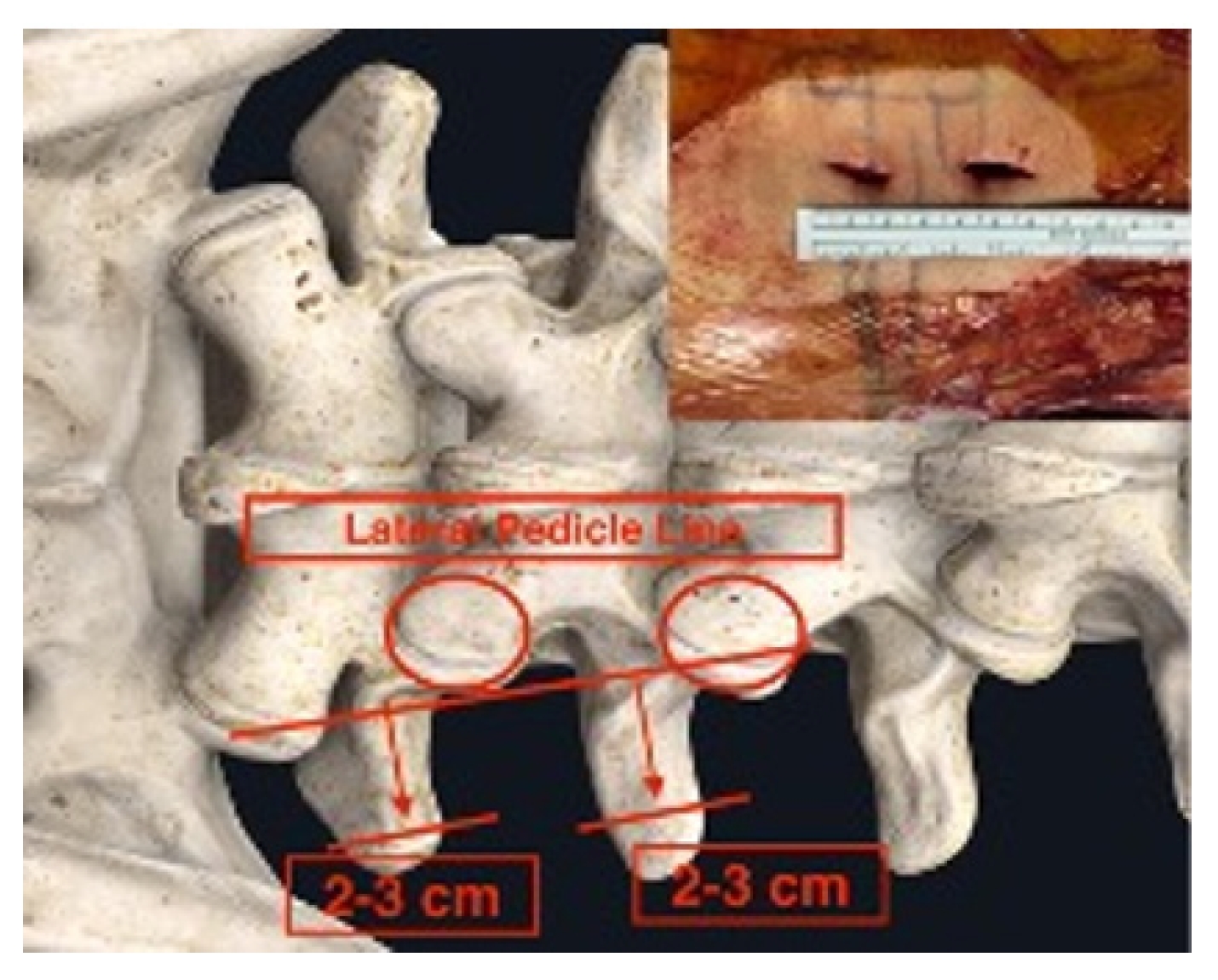
Initial skin marks were made using fluoroscopic guidance with anteroposterior and lateral view. The marks were placed over the mid-spinous line, medial pedicle line, and lateral pedicle line and contoured the pedicle shape line with further lateral marking extending 2–3 cm to the ipsilateral transverse process.
A V-line projection was created by drawing the vertical target disc line and parallel target disc line in another lateral fluoroscopic view (Figures 1, 2). This allowed for accurate plotting of the target disc coverage area.
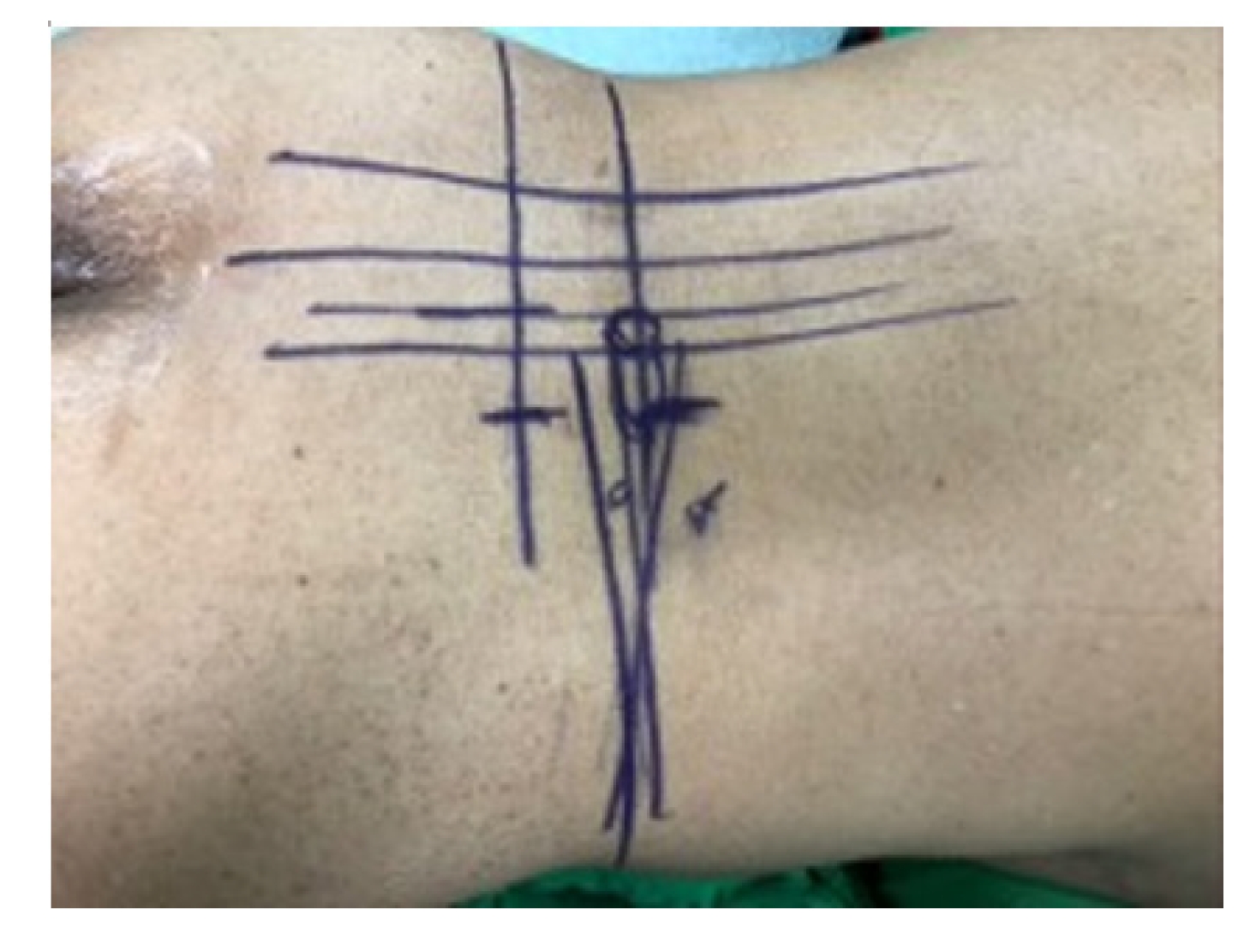
Skin tag: center spinous line, medial, lateral pedicle line, pedicle contour, transverse process extension.
3. Surgical Procedure
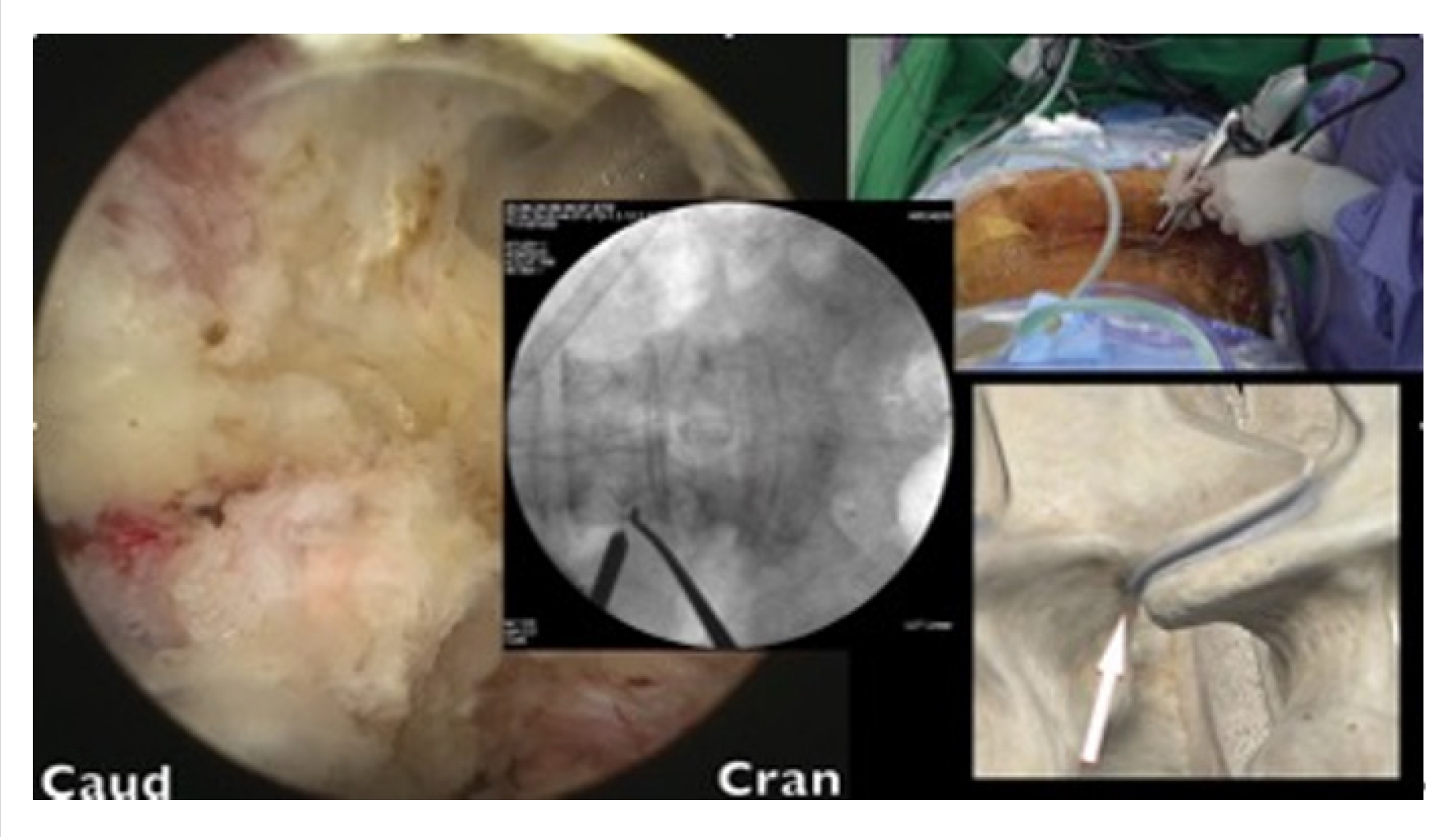
Skin incisions were made 2–3 cm to the side of the lateral pedicle line. The initial docking for triangulation was done at the base of the transverse process. After hemostasis was achieved, dissection was performed using coblators (Arthrocare, Smith-Nephew, Austin, TX, USA), moving upward toward the facet capsule. With the surgeon standing on the right side, the upward direction was targeted at the 10 o’clock position. With the surgeon standing on the left side, the upward direction was targeted at the 2 o’clock position (Figure 3). These surgical techniques have been described by Kim and Choi [10] and Park et al. [11].
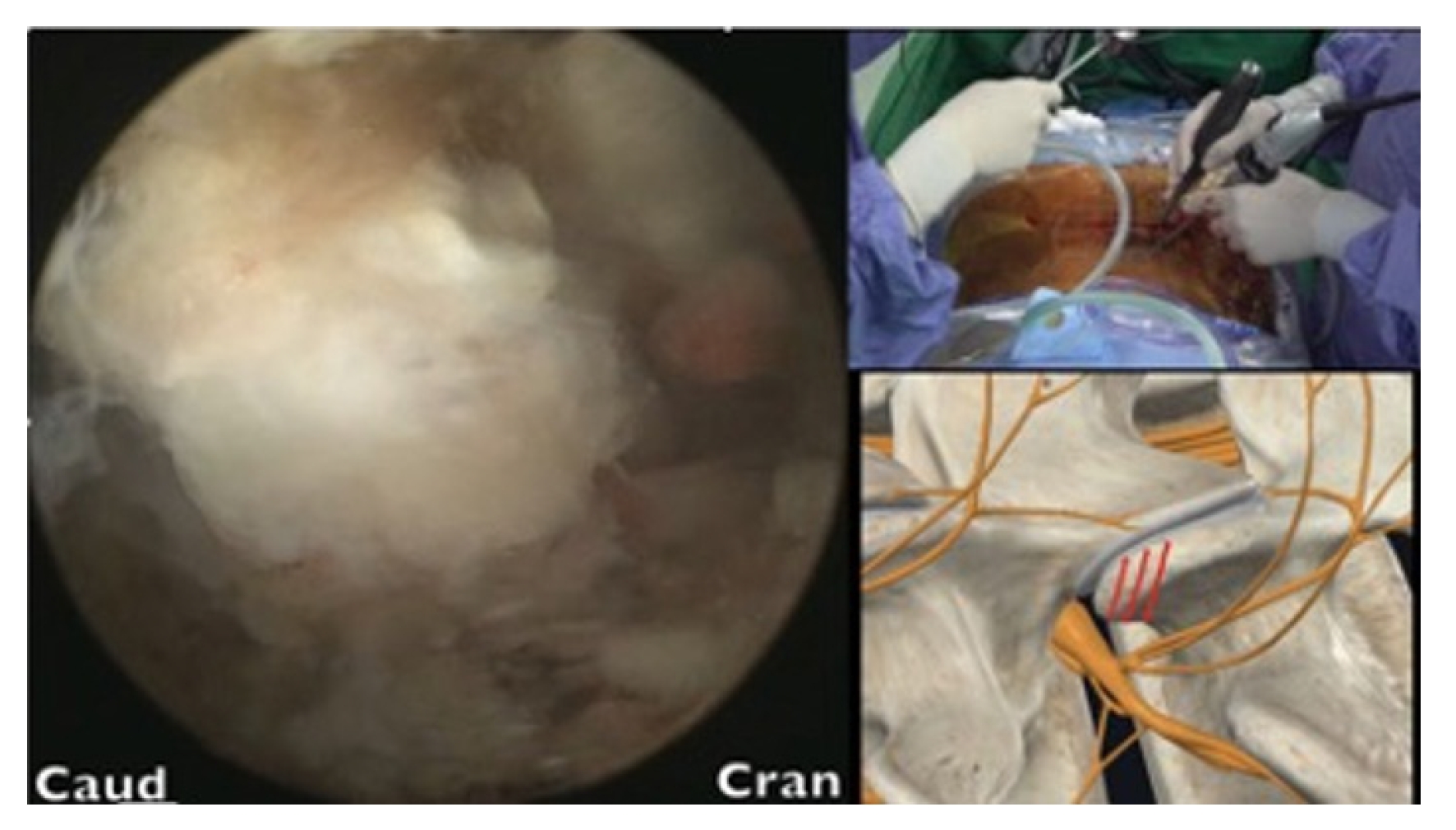
After the tip of the superior articular process (SAP) had been identified, an en block or sliced ostectomy was performed from the tip of the SAP to the upper margin of the lower pedicle, aligning with the lower end of the disc margin (Figure 4). The excised bone blocks from the SAP were then minced and used as autograft for fusion purposes.
The lumbar disc was probed using an Indian knife to determine the optimal trajectory. Once found, the disc space was dilated, and the upper and lower endplates were prepared by stripping their osteochondral surfaces. A trial cage of appropriate size was then inserted into the disc space. Autogenous bone graft from SAP ostectomy blocks mixed with demineralized bone matrix (Grafton, Medtronics) and artificial bone blocks (Bicera, Wiltrom, Hsinchu, Taiwan) were used to fill the disc space.
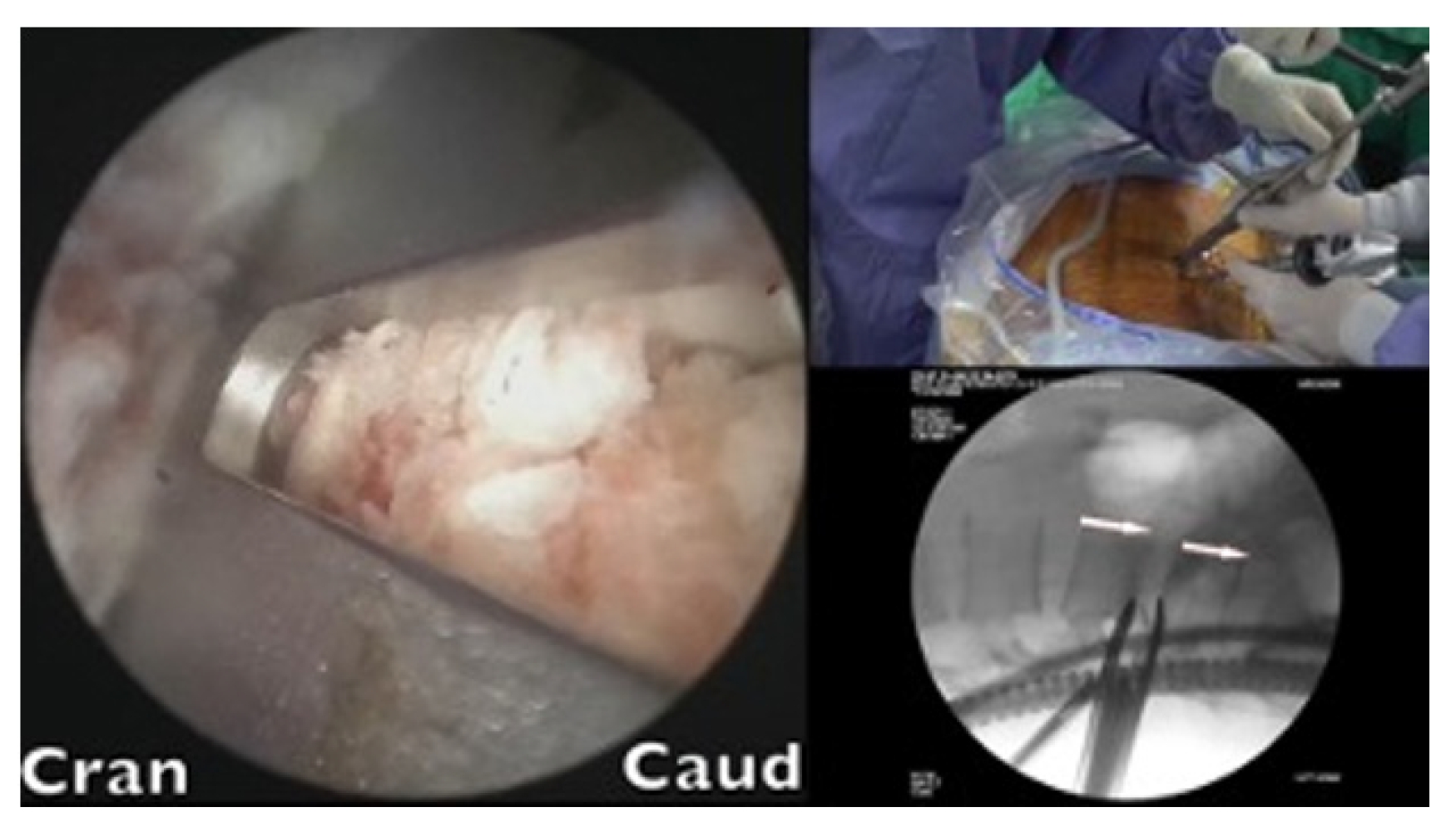
A cage sleeve glider was used to insert the cages (Figure 5). The glider was designed by the author of the present study in collaboration with Wiltrom. Arthroscopic visual and fluoroscopic guidance was employed during the procedure (Figure 6).
The procedure was presented on video clip to demonstrate the usage of the cage sleeve glider during cage insertion (Supplementary video clip 1).
The glider had 2 flanges. The windowed side enabled direct visualization of the advancement of the cage while impacting the cage holder. The closed flange, located on the exiting nerve side, served to protect nerve roots. The final seating position was determined using fluoroscopic guidance.
After the cage was inserted, the lateral recess area was ipsilaterally decompressed except in cases of severe Schiza type D spinal stenosis, in which another unilateral laminotomy with bilateral decompression (ULBD) procedure was performed beside the midline spinous process area.
RESULTS
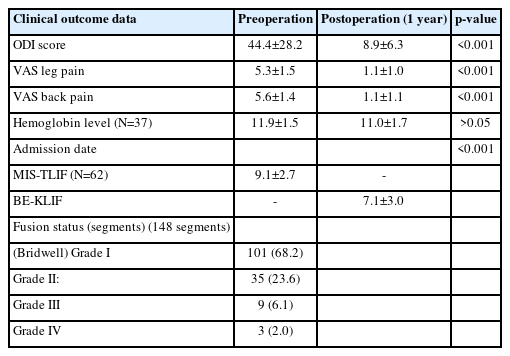
This study included 128 patients (46 men and 82 women). The median age was 69.0±13.0 years (range, 18–93 years). A total 169 segments were fused (Table 1).
The breakdown of types of fusion cases was as follows: single-segment fusion: 93 cases; 2-segment fusion: 30 cases; 3-segment fusion: 4 cases; and 4-segment fusion: 1 case.
The major diagnoses were as follows: spinal stenosis and failed back surgery syndrome (28 cases, 21.9%), adjacent segment disease (5 cases, 3.9%), tumor (1 case), and spondylolisthesis (94 cases, 73.4%). Most patients had listhetic instability with associated back and neurologic symptoms.
Fusions were performed at the following levels: L5–S1 (18 segments), L4–L5 (96 segments), L3–L4 (42 segments), L2–L3 (11 segments), L1–L2 (1 segment), and T12–L1 (1 segment).
Uniframe instrumentation was used in 57 cases, and biframe instrumentation was employed in 71 cases.
All the cages were 10 mm in width, the lengths of the cages were as follows: 26–28 mm, 4 segments (2.3%); 32 mm, 88 segments (52.3%); and 36 mm, 76 segments (45%).
Surgical time analysis revealed the following durations: first-cage surgical time (from skin incision to finalizing cage insertion): 80.0±17.1 minutes; second-cage surgical time (from skin incision for the second cage to finalizing cage insertion): 76.5±29.1 minutes; decompression time (either ipsilateral or same-side ULBD): 30.8±24.3 minutes; biframe instrumentation time (from start of incision to finalizing assembly of construct): 42.3±16.4 minutes; uniframe instrumentation time: 29.4±3.8 minutes; operating time with single-level biframe: 157.1±39.2 minutes; and operating time with single-level uniframe: 136.3±53.0 minutes.
1. Clinical Outcomes
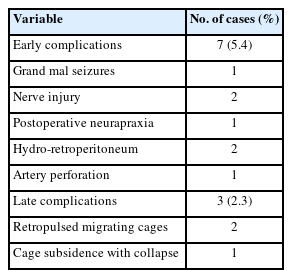
Of the 128 cases, 16 were excluded from further analysis due to loss to follow-up within 1 year (Table 2). The remaining 112 cases were followed up for a period of 12 months or more. Information on the participants’ leg and back pain levels was collected. The mean ODI score was significantly lower 1 year after the procedure than before the procedure (8.9±6.3 vs. 44.4±28.2, p<0.001). The mean VAS scores for leg and back pain were 5.6±1.5 and 5.6±1.4, respectively, before the procedure and 1.1±1.0 and 1.1±1.1, respectively, at the 1-year follow-up (p<0.001).
We selected 36 cases of single-level BE-KLIF with biframe instrumentation for an evaluation of complete blood count data during the procedure and on postoperative day 1. The average hemoglobin level was 11.9±1.5 before the procedure and 11.0±1.7 on postoperative day 1 (p>0.05). Limited data on cases involving multilevel fusion were available but not meaningful for statistic evaluation. Future studies should investigate the effects of multilevel fusion or different etiologies on hematologic data.
The mean duration of hospitalization was 7.1±3.0 days, which is much shorter than that for patients receiving MIS-TLIF procedures at our institute (9.1±2.7 days, 62 cases, 2015–2018). However, in other studies, the mean duration of hospitalization for patients receiving MIS-TLIF was 7.8±2.3 days [12] and for patients receiving BE-TLIF was 5.7±1.1 days [6]. These hospitalization durations may not be comparable due to factors such as inclusion of different etiologies, single or multilevel fusion, older age (69.0±13.0 years), comorbidities, socioeconomic factors.
In total, 16 cases were lost to follow-up after 3 months and excluded from the evaluation of fusion status. The patients underwent plain x-ray, dynamic view, or computed tomography evaluations. In total, 46 patients underwent computed tomography evaluation. Fusion status was evaluated using the Bridwell fusion grading system. In total, the fusion status for 148 segments was evaluated. Two individual clinicians evaluated fusion status. The following are the results of their evaluations: grade I: 101 segments (68.2%); grade II: 35 segments (23.6%); grade III: 9 segments (6.1%); and grade IV: 3 segments (2.0%). If we consider grades I and II to represent successful fusion, we achieved a fusion rate of 91.8%. Two cases of cage retropulsion and one case of cage subsidence and vertebrae body collapse occurred in the patients who received fusion grade IV in their evaluation. These cases were considered late complications.
2. Complications
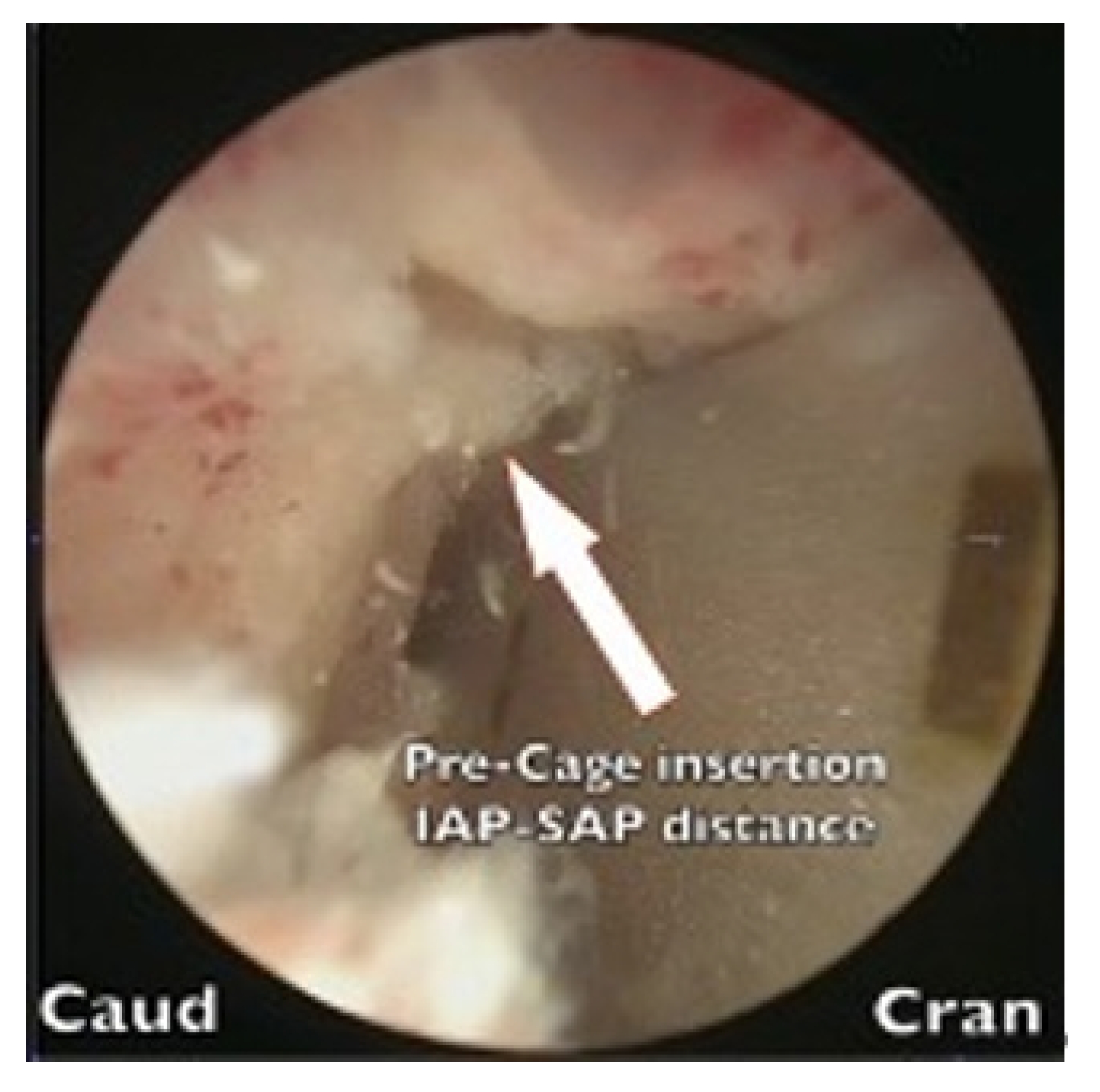
We discovered 7 cases (5.4%) of early postoperative complications (Table 3). The occurrence of hydroretroperitoneum in 2 cases was attributable to our limited experience with the far-lateral approach (FLA) during the early phase of our learning curve. A breach of the intertransversarius facia occurred, leading to leakage of irrigation fluid into the retroperitoneum. All complications were managed conservatively using diuretics and did resolve completely.
One case of tonic-clonic (grand mal) seizure cause by dura tear while preparing the cage insertion process resulted saline fluid influx into the dura canal, the patient sustained grand mal epileptic seizure attack after recovery from anesthesia on returning to ward. The seizure lasted 6 hours finally subsided by heavy sedatives. Patient had been suffered from rhabdomyolysis, acute renal failure, and pulmonary insufficiency. We resuscitated the patient, who eventually recovered after extensive cardiopulmonary care.
Two cases of neuropraxia were caused by the inadvertent use of a negative pressure suctioning shaver during disc space preparation, and one case of neuropraxia was caused by cage impingements during the insertion process.
One case of artery perforation due to instrument perforation occurred while the anterior aspect of the disc area was being prepared. The bleeding was successfully sealed off using hemostatic thrombin agents.
One patient who experienced cage retropulsion received revision surgery that involved replacing the loose cage with a new cage and performing another fusion procedure. The other patient who experienced cage retropulsion was managed conservatively due to his advanced age and poor clinical condition. One patient experienced an L1 compression fracture with a retropulsed fragment and spinal stenosis. This patient received biportal far-lateral (FLA) decompression followed by BE-KLIF. Unfortunately, this resulted in subsidence of the cage and collapse of the vertebrae at the T12–L1 junction. Due to severe kyphotic deformities and being unsuitable for further fusion, the patient was referred for PSO (pedicle subtraction osteotomy) corrective surgery.
DISCUSSION
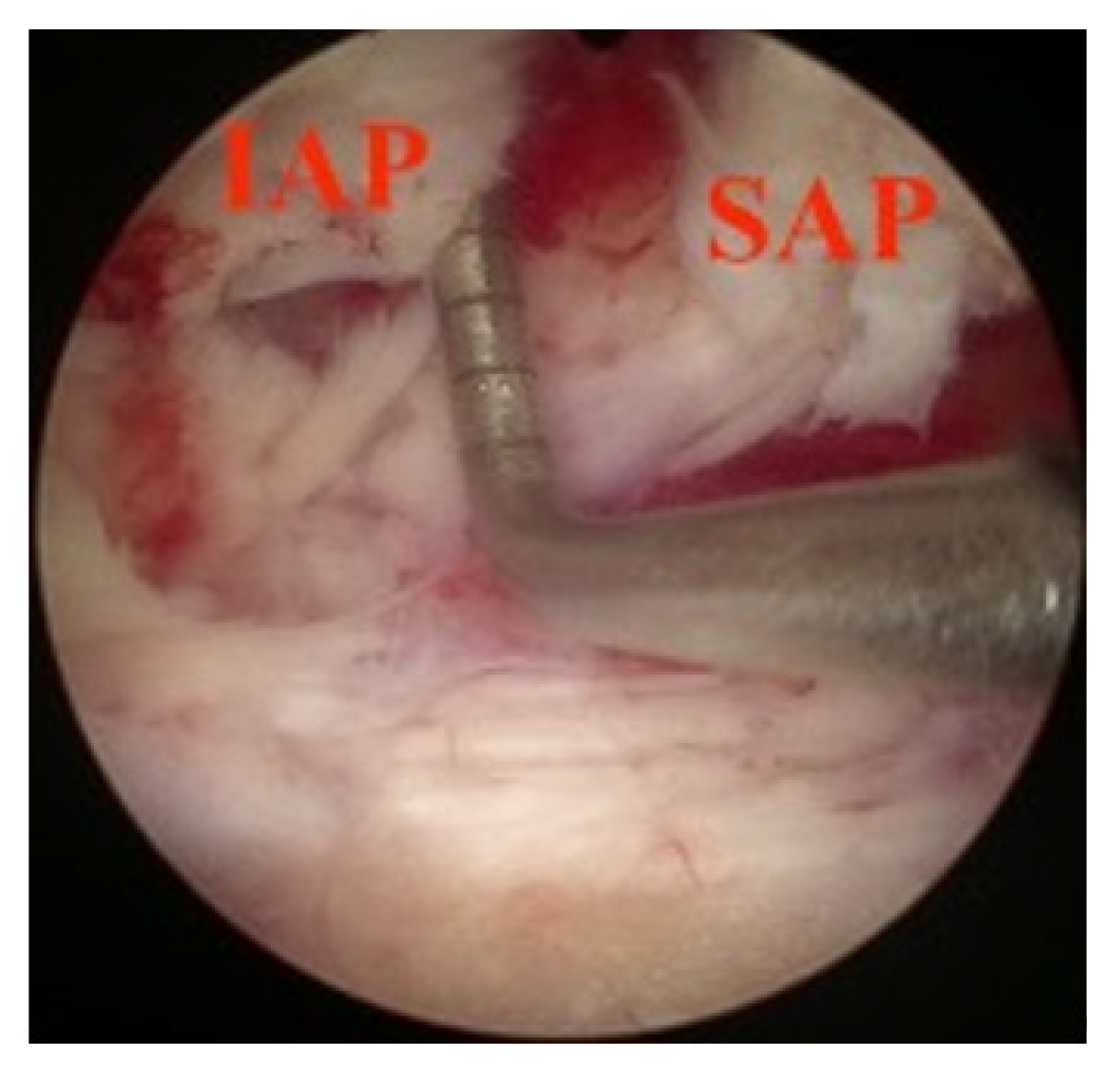
This study investigated the BE-KLIF technique, which is an alternative to BE-TLIF technique. The technique involves using a biportal endoscopic FLA, which offers several technical advantages, such as a relatively oblique trajectory, partial sacrifice of the tip of SAP, preservation of the facet joint, and the use of a large cage for direct access to the Kambin triangle for fusion purposes. Compared with the BE-TLIF, this technique allows for a more oblique trajectory (45°–60°) and enables the use of a single long cage of length 32–36 mm with potential extension to 40 mm. By using a long cage with a large effacement and encasing it at the hard apophyseal cortical rim of the vertebrae, we achieved higher disc space elevation and widening of the facet-neuroforamen corridor on both ipsilateral and supplemental ULBD contralateral endoscopic viewing (Figures 7–12).
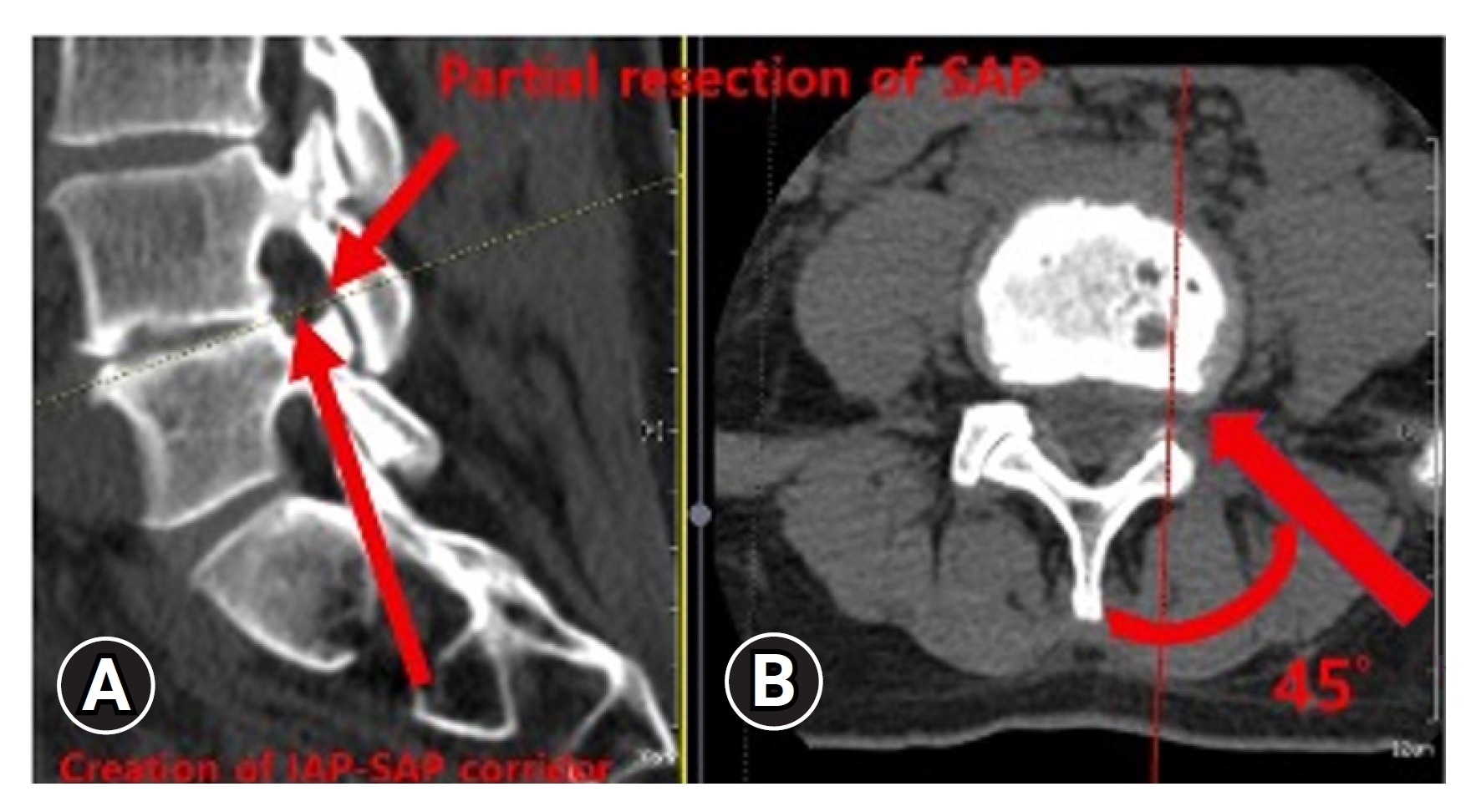
Creation of interlaminar posterior-superior articular process (IAP-SAP) corridor (red arrow)(A), 45°–60° oblique trajectory insertion angle (red arrow) (B).
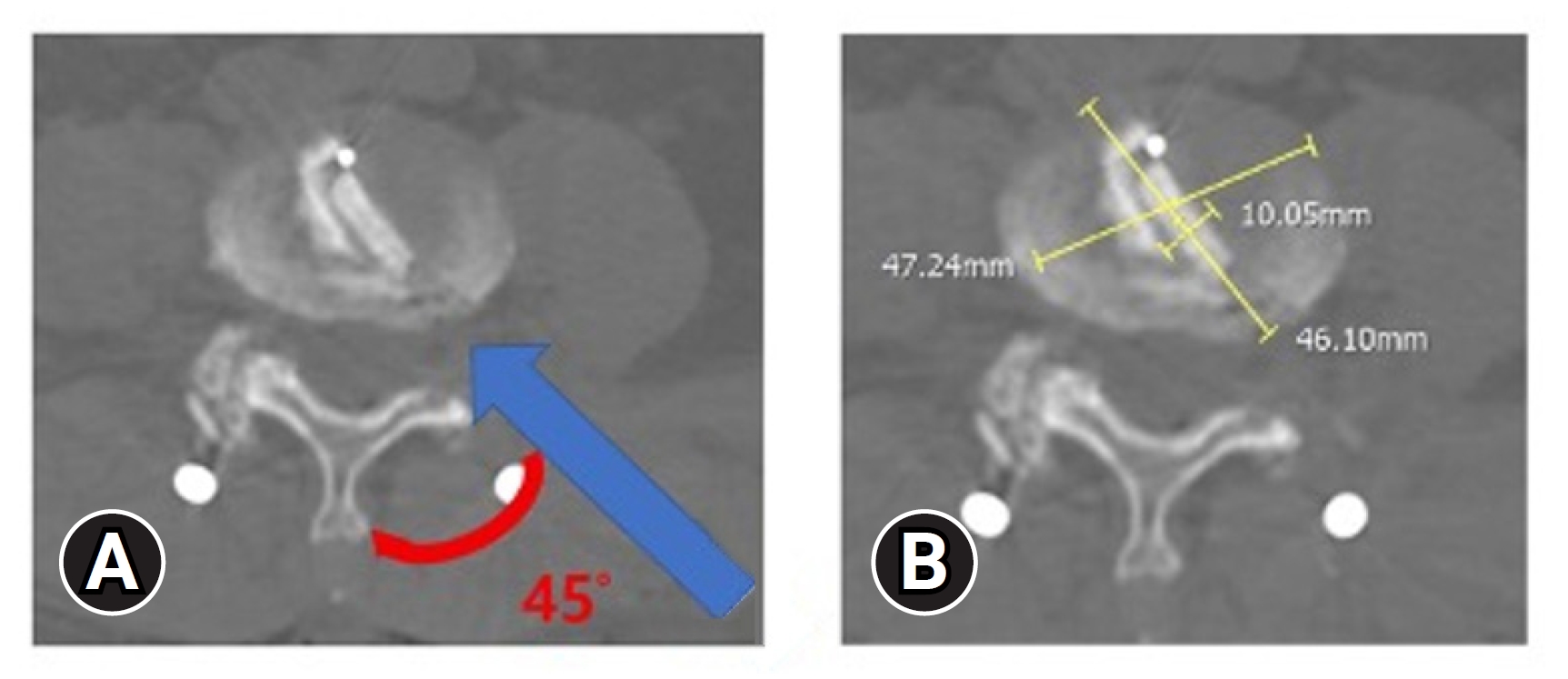
(A) Cage impregnation within the disc with 45° (red curve) oblique trajectory insertion angle (blue line). (B) Cage effacement dimension within the disc space.

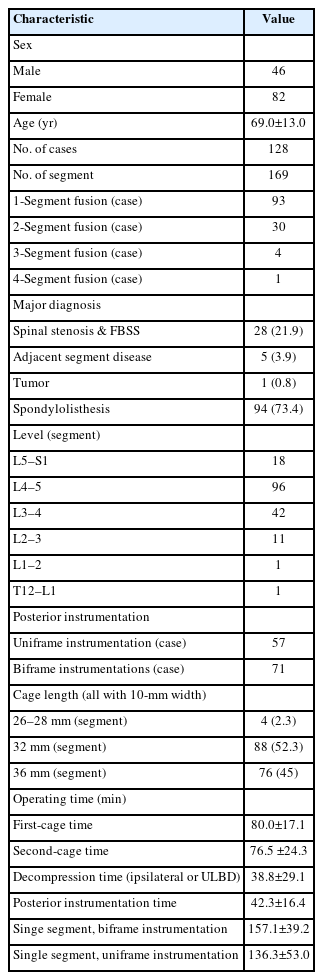
Widening of interlaminar posterior-superior articular process (IAP-SAP) distance after cage insertion.
Transverse widening of interlaminar posterior-superior articular process (IAP-SAP) distance viewed from unilateral laminotomy with bilateral decompression contralateral decompression.
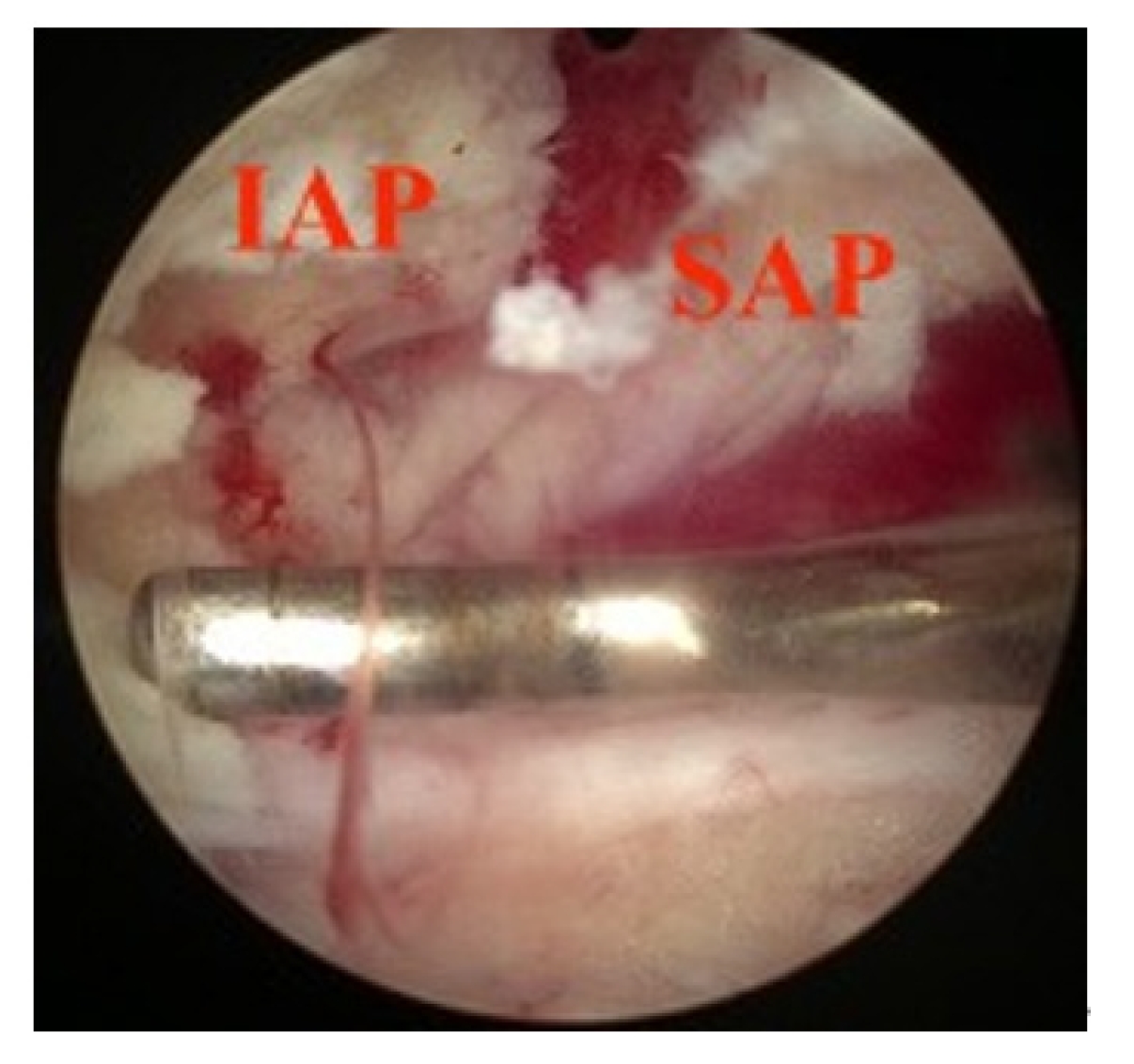
Longitudinal widening of interlaminar posterior-superior articular process (IAP-SAP) distance viewed from unilateral laminotomy with bilateral decompression contralateral decompression.
BE-TLIF uses the posterolateral approach, sacrifices the facet, and limits the trajectory and dimensions of cages. It has several advantages, including direct ipsilateral and contralateral decompression for central and bilateral neuroforamen stenosis; however, because of its proximity to central, traversing, and exiting nerve roots, it carries the risk of potential injury to nerve roots and limitation of cage size dimension. By contrast, the facet-sparing advantage offered by BE-KLIF protects the central dura trunk and traversing root with preserved bony barrier but falls short in terms of central and contralateral decompression. It relies solely on ipsilateral lateral recess decompression and indirect decompression through elevation of disc height by using a large cage. In cases of severe Schiza type D central stenosis, supplemental ULBD may be necessary.
The BE-KLIF technique requires expertise in the far-lateral (FLA) paraspinal approach, which is different from the interlaminar posterior approach (IPA) commonly used by spine surgeons. The profuse vasculature presents a challenge for bleeding control during the procedure. To access the Kambin triangle, we identified the facet capsule and located the tip of the SAP, creating an IAP-SAP corridor (Figure 7). We performed our first BE-KLIF case after completing 119 decompression cases using interlaminar posterior (IPA) and far-lateral (FLA) paraspinal approaches. Surgeon familiar with biportal endoscopic TLIF require additional FLA training transit to BE-KLIF.
Compared with our previous series of MIS-TLIF, BE-KLIF had a shorter operating time, less impact on hemostatic status, allowed for earlier ambulatory time, and resulted in shorter hospital stays. However, it did not perform as well as procedures reported in other published studies.
We observed 2 cases of hydroretroperitoneum, which we attributed to limited experience with the far-lateral (FLA) paraspinal approach. This highlights the importance of having sufficient ability to perform the procedure using a FLA and serves as a reminder that novices should use BE-KLIF techniques cautiously.
One case of grand mal seizure occurred after surgery due to a dura tear, and this case serves as a warning to abandon the procedure if the surgical time is anticipated to be prolonged. Two cases of nerve injury related to the use of a shaver serve as cautionary examples regarding the use of negative pressure suctioning shavers when preparing the disc space.
Due to the proximity of the exiting nerve root to the disc space, we recommend using a protective blunt tip cage sleeve glider when inserting large cages to prevent postoperative neuropraxia. The double-barrel cage sleeve slide acts as a buttressing shield over the inferior margin of the exiting nerve without requiring a nerve root retractor or sentinel pin.
We achieved a fusion rate of 91.8% by using the BE-KLIF fusion procedure. This is comparable to the rates reported in other studies, including 90.0% for the use of minimally invasive TLIF [13] and 93.3% reported by Pao, 78.3% by Heo, 95.1% by Park, and 93.7% by Kim for the use of BE-TLIF [6,7,14]. Our study involved 2 cases of cage retropulsion with nerve root impingement requiring revision surgery. The cage migration was likely caused using uniframe instrumentation, which provided inadequate posterior support. Additionally, one patient experienced cage subsidence leading to vertebrae body collapse at the T12–L1 level; this was caused by inadequate long posterior instrumentation and poor correction of the kyphotic angle resulting from wedge compression fracture in T12 and L1 vertebrae bodies.
CONCLUSION
BE-KLIF is a viable alternative to posterior and posterolateral endoscopic lumbar interbody fusion. The advantages of BE-KLIF include less bone removal, preservation of the facet joint, facilitation of a more oblique trajectory, potential for larger cages with wider effacements, and facet elevation for indirect decompression. However, central, and contralateral decompression is lacking in the technique. To address this concern, we recommend adding a same-side ULBD procedure to provide central and contralateral decompression. The choice of a particular fusion approach should be based on the clinician’s judgment of the patient’s condition and his expertise on FLA.
This study was a comprehensive retrospective pilot study on the BE-KLIF technique, which is not an extensively studied technique. The participants of the study comprise wide indications who had listhetic instabilities or spinal stenosis, had undergone unsuccessful back surgery, had adjacent segment disease, or had a tumor with fracture. These factors may have influenced the study outcomes.
The posterior instrumentation used after BE-KLIF (uniframe or biframe structures) may have affected the fusion rate.
All participants were operated on by a single surgeon, which may have introduced bias due to the surgeon’s learning curve. Further validation of this technique should involve multicenter studies and recruitment for more surgeon’s experience.
Supplementary Material
Supplementary video clip 1 can be found via https://doi.org/10.21182/jmisst.2023.01116.
Supplementary video clip 1.
MPEG file for demonstration of the process during insertion of cage via the cage sleeve glider.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
The author received collaborative engineering and design support from Wiltrom, Hsinchu, Taiwan. No financial transactions were involved.