Less Invasive Triangular Osteosynthesis in the Management of AO Type-B Unstable Sacral Fractures
Article information
Abstract
Objective
This prospective cohort study investigated the clinical and radiological efficacy of triangular osteosynthesis (TO) in the management of AO type-B unstable sacral fractures.
Methods
All patients with unstable AO type-B sacral fractures were included in this study. They were evaluated clinically and radiologically and underwent TO. Pre- and postoperative clinical parameters included the visual analogue score (VAS) for back pain, Oswestry Disability Index (ODI), and Gibbon classification. Radiological parameters included x-rays and multislice 3-dimensional computed tomography scans of the pelvis and the Tornetta and Matta criteria for fracture reduction.
Results
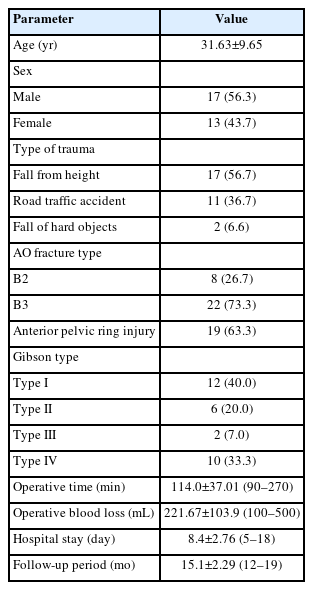
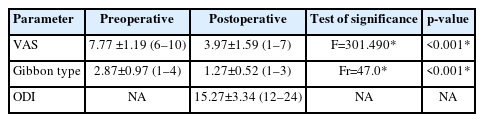
This study included 30 patients (17 males and 13 females; mean age, 31.63±9.65 years). The reported causes of trauma were a fall from height in 17 patients, road traffic accident in 11 patients, and hard objects falling onto the pelvis in 2 patients. According to the AO spine sacral fracture classification system, 8 cases were type B2 and 22 were type B3. At the last postoperative follow-up, the mean VAS improved from 7.77±1.19 preoperatively to 3.97±1.59 (p<0.001), the mean ODI was 15.27±3.34, and the Gibbon classification of cauda equina injury improved from 2.87±0.97 preoperatively to 1.27±0.52 (p<0.001). According to Tornetta and Matta criteria for fracture reduction, the results were excellent (<4 mm) in 73.3% of patients, good (4–10 mm) in 20%, and fair (10–20 mm) in 6.7%. All patients experienced complete fracture healing.
Conclusion
TO is a less invasive, safe, and effective option for the management of unstable AO type-B sacral fractures with good clinical and radiological outcomes.
INTRODUCTION
Biomechanically, the sacrum carries weights from the spine to the pelvis representing a suspensory bridge between iliac bones. It forms the posterior aspect of the pelvic ring and has therefore been described as the keystone of the pelvic ring [1].
Sacral fractures mostly occur because of high-power blunt trauma such as road traffic accidents (RTAs) or fall from height (FFH). Most of these fractures are disastrous injuries that may be associated with a high incidence of other injuries. These multiple systems injuries lead to serious morbidity and mortalit. [2,3].
The important aspects that must be evaluated while tailoring a treatment strategy are the fracture etiology, correct anatomical evaluation of the fracture, neurological condition, soft tissue status, stability, and trauma affecting other systems [4].
Sacral fractures can be treated either with conservative treatment or surgery [5]. Operative management that speeds the recovery progress and decreases the incidence of bed-ridden complications has been recommended for unstable fractures [6].
The goals of operative management are to accomplish reduction and fixation, achieve union in adequate position, restore the biomechanical stability, avoid deformity, and start rehabilitation as early as possible to achieve early return to activity [7,8]. Multiple modalities of internal fixation are available for the management of sacral fractures such as transiliac rods, iliosacral screw fixation, lumbopelvic fixation, and triangular osteosynthesis (TO) [9,10].
TO includes a combination of a vertical fixation between the lower lumbar spine and the posterior ilium on one hand, and a horizontal fixation with an iliosacral screw on the other hand. Therefore, it grants reconstruction of multiplanar stability incorporating the horizontal and vertical planes of the lumbosacral junction [11]. Compared to similar techniques, it is considered a minimally invasive technique with comparable biomechanical properties [9].
This technique has a low incidence of wound infection and soft tissue destruction compared to other techniques [11]. Cadaveric and biomechanical evaluation have shown that TO has the most biomechanically stable construct compared to other modalities of internal fixation of the sacrum [8].
This study aims to evaluate the clinical and radiological outcome of TO as a less invasive fixation technique in the management of traumatic AO type-B unstable sacral fractures.
MATERIALS AND METHODS
This prospectively designed study included patients presented to Suez Canal University Hospital Emergency Department between January 2020 to December 2021 with unilateral AO sacral fracture classification type-B [12] with minimum 12-month follow-up. Exclusion criteria were, unstable iliac fractures, first sacral vertebra comminuted fracture, fractures at iliac entry site for iliosacral screw, major psychiatric illness, pregnancy, general contraindication for surgery, pathological fractures (e.g., osteoporosis and tumors), lumbosacral transitional vertebrae.
All patients were submitted to medical history taking including, demographic data (age and sex) and mechanism of trauma. In addition, full clinical assessment was done including general examination (vital signs, complete trauma survey, and assessment of any associated soft tissue injuries), neurological assessment of lower limbs (motor, sensory, sphincters, and reflexes assessment). Back pain was assessed by visual analogue score (VAS), cauda equina injury was assessed by Gibbon classification with its 4 subtypes; type 1: none, type 2: paresthesia only, type 3: lower limb motor deficit, type 4: bowel/bladder dysfunction [13]. Also, radiological assessment included x-ray lumbosacral spine (anteroposterior [AP] and lateral views). X-ray pelvis (AP, lateral, inlet, and outlet views) and multislice 3-dimensional computed tomography (CT) scan lumbosacral spine and pelvis for typing of the sacral fracture, measurement of vertical displacement according to Tornetta and Matta [14] and identifying the anterior pelvic ring injury.
The study was approved by the Institutional Review Board of Suez Canal University Hospital (IRB No. 4270#). All patients formally consented before being scheduled for surgery. We followed the World Medical Association Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects throughout this study.
1. Surgical Technique
All patients underwent TO using unilateral open lumbo-iliac fixation and percutaneous iliosacral screw fixation, the lumbar anchoring point was L5 transpedicular screws in all patients. No patients underwent surgical decompression during the procedures. One case of associated L1 fracture underwent isolated short segment transpedicular fixation (T12-L1-L2) in addition to TO.
Surgery was scheduled as soon as the vital parameters and organ function of the patient were stable to achieve optimal preparation of the patient and improve surgical environment. The procedure was conducted under general anesthesia with patients in prone position on radiolucent operating table. All surgeries were performed by the same operative team.
In case of fracture displacement, fracture reduction was corrected by longitudinal traction done by an assistant and in case of rotation of the pelvis, was corrected with a pin inserted in the posterior iliac bone to correct mal rotation of the injured hemipelvis.
In case of anterior pelvic ring injury such as pubic ramus fracture, no direct fixation was applied, only the posterior fixation was enough.
2. Percutaneous Iliosacral Screw Fixation
Skin marking for the lateral iliosacral screw was done by drawing 2 perpendicular lines: a horizontal line at the level of greater trochanter and a vertical line at the level of anterior superior iliac spine. The entry point was 2 cm above and caudal to the point of intersection and 1-cm skin incision was made. On the lateral sacral fluoroscopic view, the entry point was in the middle of the body of the first sacral vertebra just below the iliac cortical density line. A cannulated guide was advanced into the ilium. On the lateral view, the tip of the K-wire was placed on the ideal starting spot and impacted into place with a hammer to prevent slipping. Both pelvis inlet and outlet images were obtained. After advancing the K-wire and checking its position in all views a measure was introduced over to measure the depth for proper screw length. A power drill was introduced over the wire then appropriate length cannulated screw was advanced over the guidewire under fluoroscopy. An obturator view was obtainedto ensure adequate screw impaction over iliac bone. Closure of the incision with single skin suture.
3. Open Lumbo-Iliac Fixation
Under general anesthesia and guided by operative fluoroscopy, a small (7 cm) lumbosacral ipsilateral paramedian skin incision was done. The fascia was opened paramedially, and transmuscular dissection was done to reach L5 pedicle screw entry point lateral to the superior articulation facet of L4–5 facet joint. An appropriate size poly-axial L5 pedicle screw was inserted under fluoroscopy guidance. The iliac screw entry point is dissected over the postero-medial aspect of the posterior superior iliac spine (PSIS). The inferomedial part of the PSIS was excised to create a room for the head of the screw to avoid screw prominence through the skin especially during setting.
A screw channel was cannulated in a lateral downwards tilted direction towards the ipsilateral greater trochanter between the inner and outer table of the ilium followed by placement of the iliac screw under fluoroscopy guidance above the greater sciatic notch. A connecting rod of appropriate length and proper bend was applied between L5 pedicle screw and iliac screw. L5 pedicle screws used were 6.5 mm in diameter and 45 mm in length in all cases, iliac screws were 7.5 mm in diameter with length ranged from 75 to 85 mm. Iliosacral screws were cannulated 7.3-mm screws with length ranged from 80 to 100 mm. Copious saline irrigation was done followed by wound closure in layers with closed suction drain (Figures 1–3).
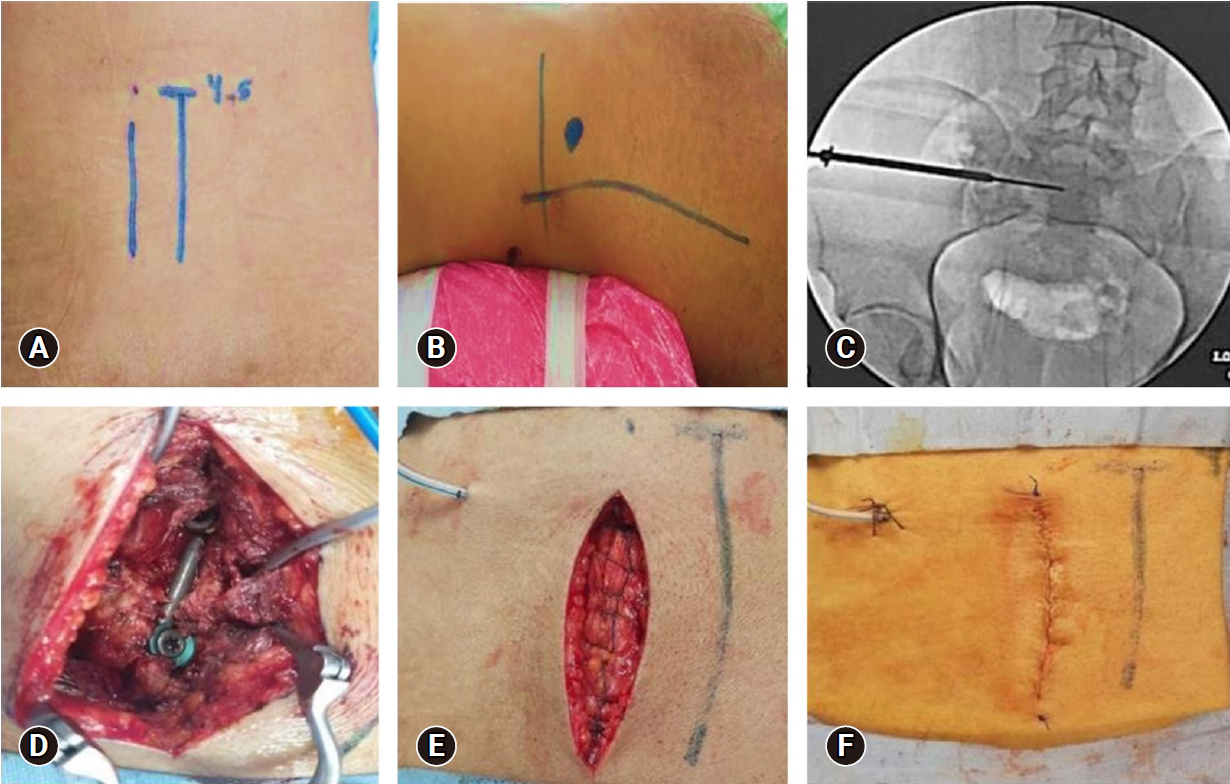
Operative images of a 30-year-old male patient who presented after a hard object fell onto the pelvis (Gibbons type I and AO type B3-N0-M3). (A) Identification of the midline and paramedian skin incision. (B) Skin marking for the iliosacral screw by drawing 2 perpendicular lines (a horizontal line at the level of the greater trochanter and a vertical line at the level of anterior superior iliac spine). The entry point was 2 cm above and caudal to the point of intersection. (C) Fluoroscopy image pelvic inlet view showing iliosacral screw insertion over K-wire and (D) the L5 screw and iliac screw connected by the rod. (E) Closure of the lumbosacral fascia with a continuous absorbable suture. (F) Skin closure with a continuous subcuticular absorbable suture.
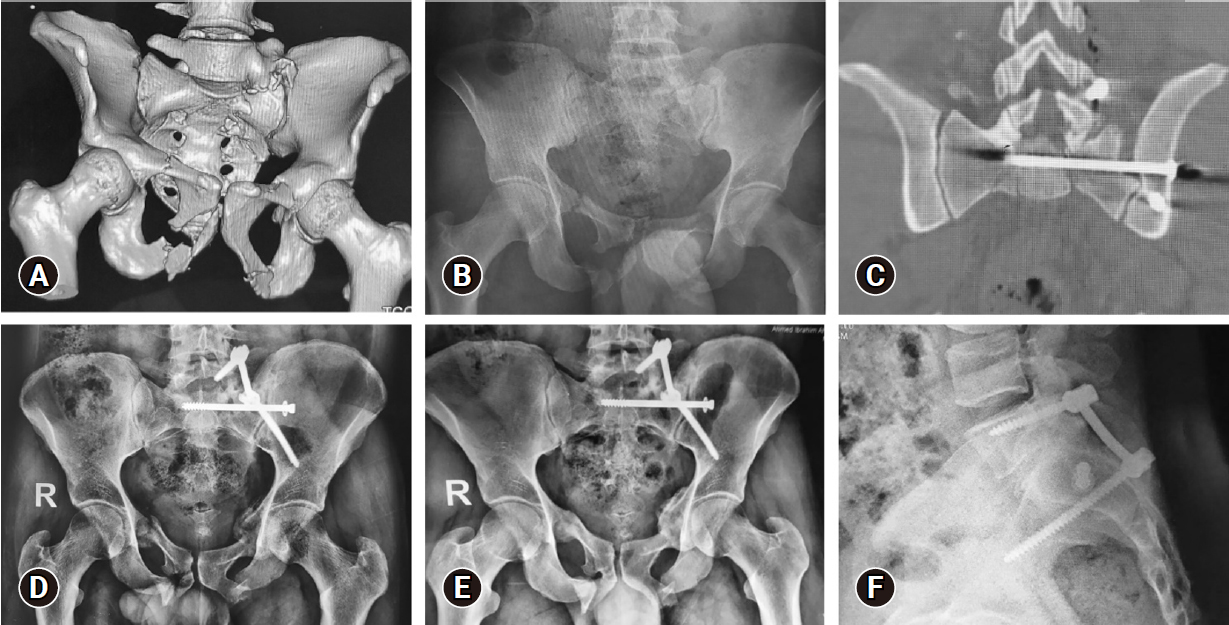
Images of the same patient as in Figure 1. (A) Three-dimensional multislice computed tomography (MSCT) scan. (B) Anteroposterior (AP) plain radiograph showing left-side AO type B3-N0-M3 sacral fracture, and associated bilateral superior and inferior pubic rami fractures. (C) Coronally reformatted MSCT scan showing the iliosacral screw in position. (D, E) AP plain radiographs showing adequate alignment and bone healing at 3 and 12 months respectively. (F) A lateral radiograph showing an adequate construct at 12 months.
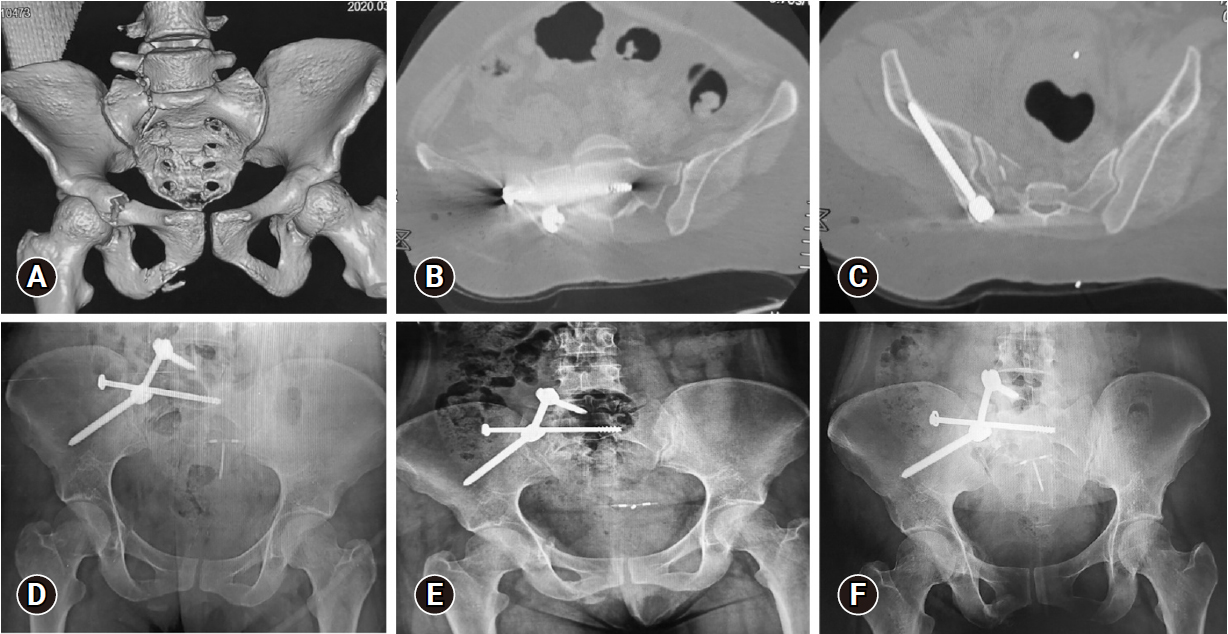
Images of a 33-year-old female patient who presented after a fall from a height, Gibbus I. (A) Three-dimensional multislice computed tomography (MSCT) scan showing right side, AO type B3-N0-M3 sacral fracture, with the following associated injuries: T12 fracture, L5 transverse process fracture, and superior and inferior right pubic rami fractures. (B) Axial MSCT image showing the iliosacral screw in position. (C) Axial MSCT image showing the right iliac screw in position. (D–F) Anteroposterior plain radiograph showing adequate alignment and bone healing at 3, 6, and 12 months respectively.
4. Postoperative Management
Operative details were recorded including length of back incision, operative time, operative blood loss, operative complications, and hospital stay.
Postoperative medications include 48 hours of intravenous (IV) 3rd generation cephalosporine antibiotics and IV analgesics. Immediate postoperative full neurological assessment for any added deficit was done. Patients started ambulation on the first postoperative day (if not contraindicated due to other injuries). Patients were allowed to bear weight and sit as tolerated.
5. Follow-up
According to follow-up protocol, patients were followed at 3 months postoperative then at 3 months interval for at least 12 months after surgery. At each visit the following parameters were reported: clinical parameters included VAS for back pain, neurological examination, Gibbon classification of cauda equina injury and Oswestry Disability Index (ODI). Radiological parameters included: x-ray lumbosacral spine: AP and lateral views, x-ray pelvis: AP, lateral, inlet, and outlet views. Multislice 3-dimensional CT scan lumbosacral spine and pelvis were performed at 6-month follow-up and if there would have been an event that requires rescanning. Fracture healing was evaluated by presence of connecting bony trabeculae and callus formation. Presence of radiolucency or loss of reduction is suggestive of loosening and implant failure.
RESULTS
Out of 36 patients recruited for this study, a total of 30 patients who completed a minimum of 12-month follow-up were reported. According to AO Spine sacral fractures classification system, 22 patients were type B3 and 8 patients were type B2. Anterior pelvic ring injury, pubic rami fractures were reported in 19 patients (63.3%). Table 1 summarizes patients’ data. Preoperative neurological assessment revealed that 12 patients (40%) were intact, 18 (60%) have sensory deficit in lower limbs, 12 (40%) have motor deficits in lower limbs, and 10 (33.3%) have saddle area hypesthesia/anesthesia. According to Gibbon classification of cauda equine injury, 12 patients were Gibbson I, 6 were Gibbson II, 2 were Gibbson III, and 10 were Gibbson IV (Table 1).
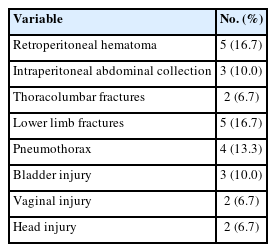
The reported associated injuries included: retroperitoneal hematoma in 5 patients (16.7%), intraperitoneal abdominal collection in 3 patients (10%), other spine injures including T 12 fracture, L 1 fracture and lumbar transverse processes fractures in 2 patients (6.7%), lower limb fractures in 5 patients (16.7%), pneumothorax in 4 patients (13.3%), bladder injury in 3 patients (10.0%), vaginal injury in 2 patients (6.7%), and head injury in form of skull fissure and intra cranial hemorrhage in 2 patients (6.7%) (Table 2).
The meantime till surgery was 5.87±2.45 days (range, 3–14 days). The mean length of the skin incision was 7.1±0.99 cm (range, 6–9 cm). The mean operative time was 114.0±37.01 minutes (90–270 minutes), the mean operative blood loss was 221.67±103.9 mL (range, 100–500 mL). the mean hospital stays was 8.4±2.76 days (range, 5–18 days), the mean follow-up period was 15.1±2.29 months (range, 12–19 months) (Table 1).
1. Radiological Outcome Assessment
All our cases demonstrated fracture healing and bony union. No implant breakage or backing-out were reported over the follow-up period. No radiolucency around implant was detected. According to Tornetta and Matta criteria for fracture reduction, the preoperative fracture displacement was <4 mm in 5 cases (16.7%), 4–10 mm in 10 cases (33.3%), 10–20 mm in 8 cases (26.7%), and > 20 mm in 7 cases (23.3%). Postoperatively, the results were excellent (<4 mm) in 22 cases (73.3%), good (4–10 mm) in 6 cases (20.0%), and fair (10–20 mm) in 2 cases (6.7%).
2. Functional Outcome Assessment
At the last postoperative follow-up, the mean VAS improved from 7.77±1.19 (range, 6–10) preoperatively to 3.97±1.59 (range, 1–7) (p<0.001), the mean ODI was 15.27±3.34 (range, 12–24), and the Gibbon classification of cauda equina injury improved from 2.87±0.97 (range, 1–4) preoperatively to 1.27±0.52 (range, 1–3) (p<0.001) (Table 3).
Reported complications were 1 patient developed pelvic retroperitoneal hematoma postoperative that was not present on preoperative pelvi-abdominal CT scans, mostly due to misdirected K-wire breaching the anterior border of the sacrum and mostly injuring the presacral venous plexus. The condition was diagnosed immediately postoperative as the patient developed hypovolemic shock. Maximum hematoma diameters were (10 cm, 8 cm, 6 cm). Patient was managed conservatively with multiple follow-up pelvi-abdominal ultrasound and CT scan and keeping the patient vitally stable using IV fluids and blood transfusion. Patient was discharged on day 6 postoperative.
Two patients had misdirected percutaneous iliosacral screw breaching the neural canal, which did not lead to added motor deficit and resulted in added sensory dysesthesia along S1 and S2 dermatomes. In 1 patient, the pain did not respond to medical treatment and the patient underwent another surgery to remove the iliosacral screw 3 months after surgery, and pain improved after. In the other patient, the pain was responsive to medical treatment and no revision surgery was needed. No other complications were recorded.
DISCUSSION
Sacral fractures are one of the common and could be disabling clinical conditions with a major socioeconomic burden. Various therapeutic modalities could be offered to those patients. In this prospective cohort study, we reported a total of 30 patients were recruited for this study including 22 patients type B3 and 8 patients type B2 according to AO Spine sacral fractures classification system. All patients were managed with TO. The preoperative VAS and Gibbon classification of cauda equina injury improved from 7.77±1.19 to 3.97±1.59 and 2.87±0.97 to 1.27±0.52 respectively at the last follow-up.
The mean age in our study was 31.63 years which corresponds to similar studies reporting TO [4,11,15-17]. Other epidemiological studies [18,19] attributed this age incidence to reckless activities and concluded that trauma is a pathology of the young. Males represent 57% in our study which is close to the work of Schildhauer et al. [11], while in the study of Erkan et al. [4] males represent 37% of cases. This difference may reflect the socioeconomic background of patients reported.
All cases suffered high-energy trauma, which leads to multiple organ injuries including neurological affection. The reported causes of trauma were FFH in 56.7%, RTA in 36.7%, and fall of hard objects on pelvis in 6.6% of cases, which was close to the work of Erkan et al. [4]. This figure was different from the study of Jindal et al. [15] conducted in India and reported that 82% of cases were due to RTA which could be explained by the fact that India has highest worldwide percentage of RTA deaths [20]. In our study, 59% of FFH where females and 82% of RTA were males. Epidemiological studies [19,21] reported that males were affected by trauma more than females and the most common injury among males was RTA while females were mostly victims of FFH. This data could explain variations in gender presentation and its relation to the mechanism of trauma.
Anterior pelvic ring and pubic rami fractures were reported in 63.3% in our study which corresponds to other studies [4,15,22], meanwhile reported associated injuries in our patients were diverse and close to other reports in the literature [4,15,22]. In the work of Schildhauer et al. [17] the most common associated injury was lower limb fractures. In a prospective study analyzing 100 patients with pelvic fractures, Lunsjo et al. [23] reported that the associated injuries (evaluated by the injury severity score) and not fracture stability were the most important predictors in defining mortality in these patients. The same results were found by Parreira et al. [24] in their study to evaluate the role of associated injuries on outcome of patients with pelvic fractures, which reported 103 patients. They concluded that the patient's outcome correlates with the severity of the associated injuries rather than the fracture pattern.
Twelve of our patients (40 %) had motor neurological deficit which was close to the work of other studies [2,11,16,17,25] who reported that 65%, 52%, 59%, and 57% of their patients had neurological deficit respectively. In our study, the mean pre- and postoperative Gibbon scores were 2.87 and 1.37 respectively with 52% improvement. This corresponds to the literature such as work of Hu et al. [16] with 3 and 1.8 mean pre- and postoperative Gibbon score respectively, and the work of Erkan et al. [4] with 2,7 and 1,3 mean pre- and postoperative Gibbon score respectively.
In our study, the mean period from trauma till surgery was 5.87 days (range, 3–14 days). A similar figure of 13 (range, 0–23), 9.7 (range, 3–21), 9 (range, 1–17), and 13 days (range, 0–28 days) days were reported by Schildhauer et al. [17], Jindal et al. [15], Mouhsine et al. [25], and Schildhauer et al. [11] respectively.
The postponement in the surgical intervention was attributable for optimization of the patients' homeostatic and physiological conditions and time taken for healing of any soft tissue injuries in the surgical field. According to a systematic review published in 2017 [26] that reported 30 articles and 309 patients to evaluate the effect of formal laminectomy and timing of surgery for patients with sacral fractures and neurologic deficit on clinical outcome, they reported no benefit of early surgery within 72 hours of trauma.
On the other hand, Routt et al. [27] in their series reported that surgery postponement more than 5 days were linked to weaker closed reduction percentage. Another series by Alaswad et al. [28] reported cases that were operated in the first 7 days had a higher percentage of wound healing problems compared to the cases that were operated later. This was attributed to the presence of soft tissue edema and less optimization of the general condition. They also reported no difference in sphincter and/or neurological injuries improvement whether the surgery was done early or late after trauma.
In this series, we did not have any cases of loss of reduction, implant breakage or nonunion which we attributed to multiple technical details in our surgical procedure. Fracture reduction was performed by applying longitudinal traction by an assistant and maintaining it till inserting iliosacral screw, which is applied first before lumbopelvic fixation. If the lumbopelvic fixation was applied at the beginning; this would prevent fracture compression and closure of fracture gap when the iliosacral screw was applied afterwards.
According to Tornetta and Matta criteria for fracture reduction, we reported excellent results in 73.3%, good results in 20% and fair results in 6.7% of patients. Hu et al. [16] reported excellent results in 72%, good results in 24% and fair results in 4% of patients.
Jindal et al.[15] reported fracture union and no loss of reduction in 21 out of 22 cases. Hu et al. [16] reported fracture union, no implant loosening or breakage in all 22 cases. Also, Mouhsine et al. [25] reported fracture union, no loss of reduction and no hardware loosening in all cases. In all the previous studies, iliosacral screw was applied at the beginning. On contrary, Sagi et al. [22] reported 8% percentage of nonunion which was attributed to the surgical technique that lumbopelvic fixation was applied before iliosacral screws which leads to inadequate compression of the fracture with the iliosacral screw.
Formal decompression and laminectomy even in the presence of neurological deficits is a controversial issue with multiple contradicting studies. We did not perform any decompression in this study as we considered that the neural injury is more related to the impact and shearing effect of trauma rather than neural compression. We relied mainly upon fracture reduction to help sacral alignment and improve neural injury recovery. Schmidek et al.[29] recommended early decompression in his study, which included 11 patients with transverse sacral fractures. Schildhauer et al. [30] reported better results for decompression and observed that 15 of 18 patients (83%) with a U-type sacral fracture with complete bowel and/or bladder dysfunction had some degree of neurological improvement after sacral laminectomy and lumbopelvic fixation. Erkan et al. [4] performed laminectomy on 5 patients in his study, which included 19 patients and reported superior neurologic outcomes. On the other hand, Sagi et al. [22] did not do laminectomy in his study with included 58 patients and reported good outcomes. Nork et al. [10] reported improvement in neurological status in 7 patients who underwent iliosacral screw fixation without laminectomy. Elhabashy et al. [7] also reported similar results on 20 patients with sacral fractures who underwent iliosacral screw fixation without laminectomy. Jindal et al. [15] did not perform laminectomy in his study and reported neurological improvements. In the work of Hu et al. [16], 13 patients underwent laminectomy with diverse outcomes that did not show any benefit of laminectomy. According to a systematic review published in 2017 [26], it does not have any benefit regarding improvement in neurological functions. This review also showed that neurological impairment is mainly because of crushing and shearing of the neural tissue rather than compression.
In this series, 1 patient had postoperative retroperitoneal hematoma and 2 had maldirected iliosacral screw with neural canal breaching. No one had wound infection or healing problems. This may be attributed to our less invasive technique as previously detailed, also adequate submergence of iliac screw head below the profile of posterior iliac crest by excising the inferomedial part of the PSIS to create a room for screw head which decreases screw prominence and leads to less tissue necrosis and less wound healing problems.
Schildhauer et al. [17] reported in 48 patients' series, 1 case of pulmonary embolism leading to death, 3 cases of tissue necrosis overlying iliac screw head requiring revision, and 3 cases of infection requiring implant removal. Jindal et al. [15] reported in 22 patients' series, 3 cases of wound infection with debridement in one and 2 patients of connecting rod back out. Hu et al. [16] reported 2 out of 22 patients with wound infection treated conservatively. Mouhsine et al. [25] reported a case of wound infection that needs implant removal of 7 patients. Erkan et al. [4] reported 26.3% wound infection rate that may be attributed to the midline skin incision with very large surgical field and excessive muscle dissection and devitalization. They also reported that wound healing problems increase in cases with degloving soft tissue injury.
Less invasive TO is a unique technique in a way that it combines both percutaneous fixation and mini-open minimally invasive techniques. In our study, iliosacral screw was inserted percutaneously and lumbopelvic system was applied unilaterally using paramedian skin incision and transmuscular dissection which leads to smaller surgical field, less tissue devitalization, less muscle injury, less operative time, and less blood loss. These technical advantages improve the clinical outcome and recovery, decrease wound infections and morbidities, and facilitates early rehabilitation and immediate weight bearing and early return to normal daily activities and work. The drawbacks and limitations of our described TO includes its indication in unilateral sacral fractures, and does not allow open fracture reduction.
We recommend that during TO, the iliosacral screw should be applied before lumbopelvic system to allow fracture reduction, attention must be paid to soft tissue injury and submergence of iliac screw head, and it can be performed for unstable sacral fractures in the presence of other injuries that prohibit early weight bearing as it allows safe mobilization during nursing care and decreases back pain.
Limitations of this study include a small sample size and representing a single spine center. Also reported data is not supported by biomechanical parameters and lacks a control group for comparison. However, being a prospective study with a homogenous group of patients treated with the same surgical technique and their classification according to the newly lunched and evaluated AO spine sacral trauma classification are strength points.
CONCLUSION
Our results suggest that TO is a safe and effective method in treatment of sacral fractures type-B AO Spine sacral fracture classification. It is a stable fixation construct that allows early weight bearing with good clinical and radiological outcomes and low complication rate through our 1-year follow-up period.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.