Minimally Invasive Surgery vs. Open Surgery for Infectious Spondylodiscitis: A Systematic Review and Meta-Analysis
Article information
Abstract
Objective
Minimally Invasive Spinal (MIS) Procedure has long been used for treating degenerative spinal disorders, however its usage for infectious diseases of the spine has not been described a lot in literatures. Through this meta-analysis, we aim to objectively describe the efficacy of MIS as compared to traditional open surgery (OS) in treating infectious spondylodiscitis.
Methods
A systematic search was conducted based on PRISMA guideline to identify relevant studies through PubMed, Google Scholar, and Cochrane database. A total of 4 studies (301 patients) were included, divided into 8 meta-analysis, processed using Review Manager 5.3.
Results
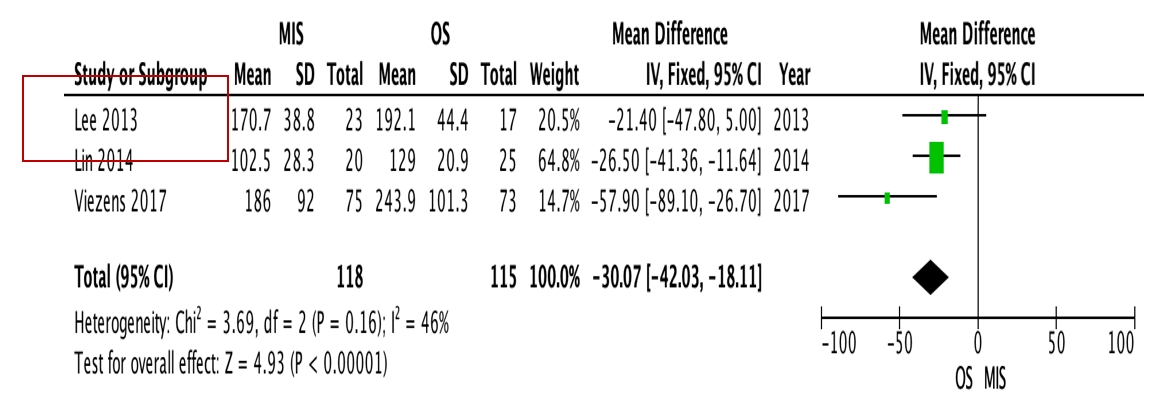
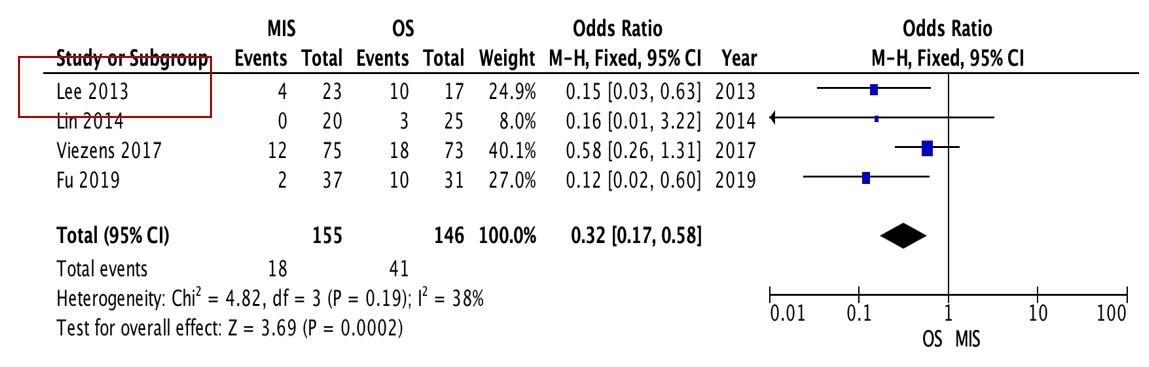
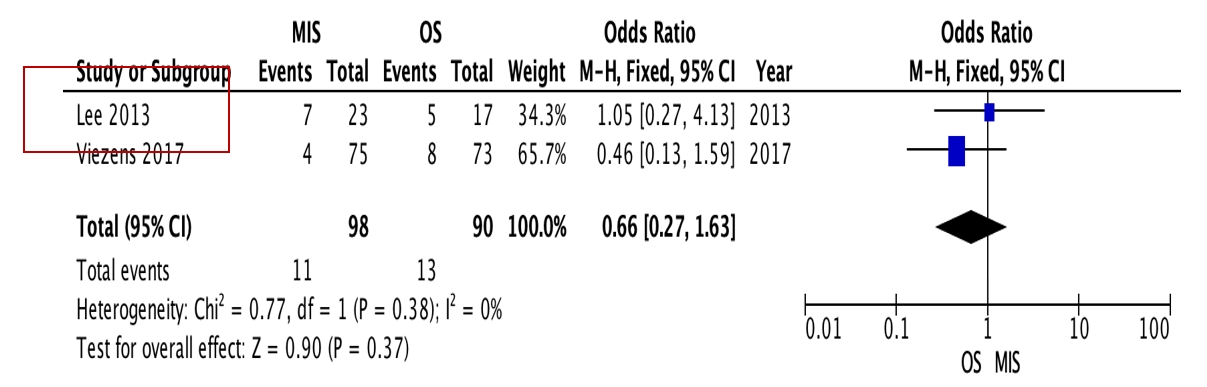
OS requires significantly longer hospital length of stay (p=0.0009, I2=0%, MD=–6.64) and higher blood loss (p<0.00001, I2=40%, MD=–264.31) as well as more postoperative blood transfusion (p<0.00001, I2=0%, MD=–1.58). Moreover, MIS has benefit in significantly shorter operation time (p<0.00001, I2=46%, MD=–30.07) and less complication rate (p=0.0002, I2=38%, MD=0.32). However, the two procedures do not differ significantly in terms of neurological improvement, recurrence rate, and mortality rate.
Conclusion
Current systematic review and meta-analysis suggest that MIS offers comparable efficacy as well as less hospital length of stay, blood loss, operation time, and complication rate compared to OS.
INTRODUCTION
Infectious spondylitis is estimated to affect 4–24 patients per million per year, with male:female ratio of 1.5:2.1. Contributing to 0.15%–5% of all osteomyelitis cases, until date, the treatment of infectious spondylitis is still very challenging in order to minimalize the mortality and morbidity of these patients [1,2].
Surgical intervention is needed in patients unresponsive to antibiotic treatments, those with spinal instability, neurological deficit, soft tissue abscess involvement, and significant spinal destruction. In order to ensure infection eradication, open surgery has been used more commonly. However it has been related to postoperative complications and morbidity, especially in immunocompromised and vulnerable patients [3,4].
As a developing method in spine surgery, Minimally Invasive Spinal (MIS) Procedure has long been used for treating degenerative spinal disorders. However, over the years its usage has been extended for other pathologies. Unfortunately, its usage for infectious diseases of the spine has not been described a lot in literatures. Compared to traditional open surgery, MIS offers less duration of surgery, less muscular trauma, and shorter hospital stay, making it a potential treatment method, especially in high-risk patients with infectious spondylitis [1].
Until now, there has not been any consensus thoroughly describing the difference between MIS and OS as the treatment for infectious spondylitis. Through this meta-analysis, we aim to objectively describe the efficacy of the two procedures in treating this pathology.
MATERIALS AND METHODS
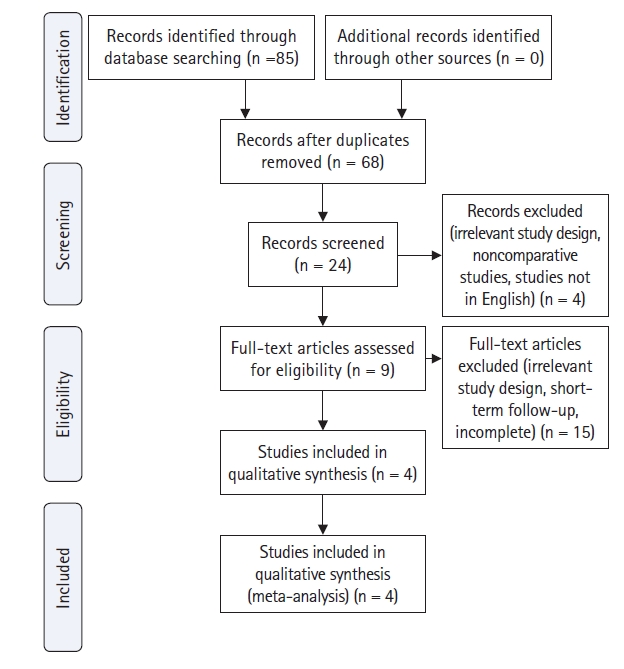
The study design was a systematic review and meta-analysis of relevant comparative studies. A systematic search was conducted from September 2020 to April 2021 to identify relevant studies through PubMed, Google Scholar, Cochrane, Medline, and EMBASE Database based on PRISMA guidelines (Figure 1). The keywords used were: “Minimally Invasive Spine Surgery” AND “Open Surgery” AND (“Spondylodiscitis” OR “Spine Infection” OR “Spondylitis”) AND “Efficacy”.
Though there has been no strict definition of Minimally Invasive Spine Surgery, but McAfee et al. (2010) [5] defined MIS as a surgical technique resulting in less tissue damage, decreased morbidity, and faster functional recovery than traditional open surgery, without differentiation in intended surgical goals, in which several terms are encompassed, such as “mini-open”, “tubular”, or “percutaneous”.
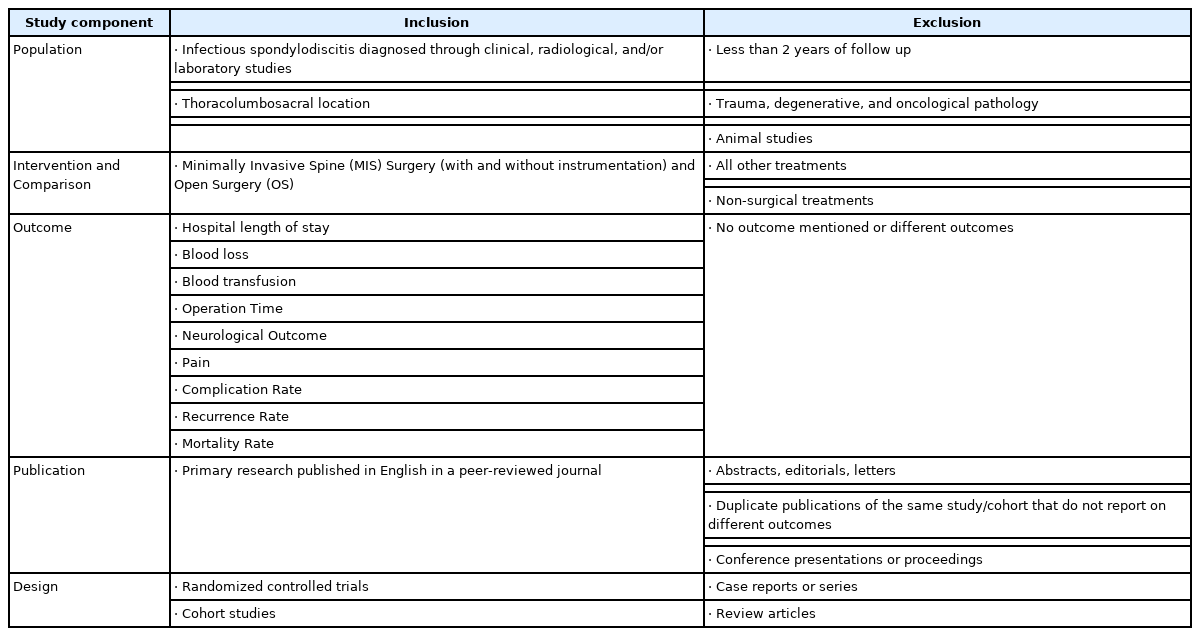
Those studies were then manually scanned and reviewed by all authors according to the following inclusion criteria: (1) minimally invasive spinal surgery and open surgery were interventions under comparison; (2) the population included patients with infectious spondylodiscitis diagnosed through clinical, radiological, and/or laboratory studies, in thoracolumbosacral location; (3) at least one of the following outcomes was reported: hospital length of stay, blood loss, blood transfusion, operation time, neurological outcome, pain, complication rate, recurrence rate, mortality rate, (4) the study was published in English, and (5) applied a randomized controlled trial (RCT) or cohort study design. The exclusion criteria were: (1) less than 2 years of follow up, (2) trauma, degenerative, and oncological pathology, (3) animal studies, (4) case reports or series, review articles, and noncomparative studies are also excluded. Table 1 presents the inclusion and exclusion criteria according to the PICO method (Population, Intervention, Comparison, and Outcome).
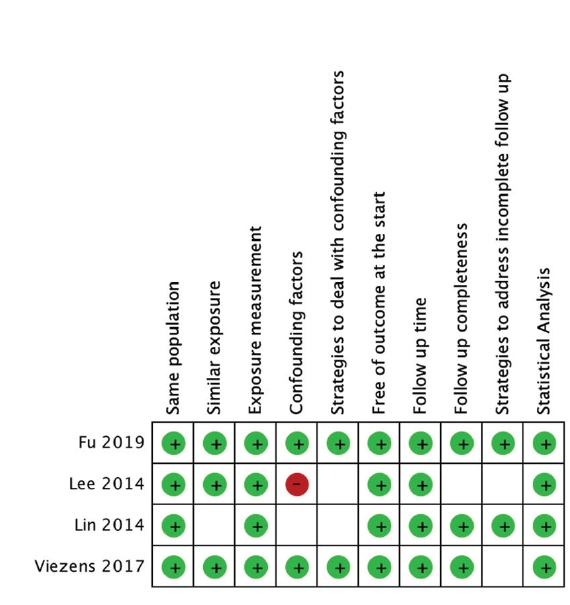
Of all potential studies, critical appraisal was performed to assess the eligibility of those studies using a scoring system adapted from Joanna Briggs Institute (JBI), comprising 10 aspects from the view of population, exposures, confounding factors, outcome, follow-up, and statistical analysis. From each included study, data related to patient and study characteristics (e.g. age, sex, level of pathology, causative organisms) and outcomes were extracted and aggregated. Continuous variables — hospital stay, blood loss, blood transfusion, operation time — were compared in terms of weighted mean difference (WMD). Dichotomous variables — neurological improvements, complication rate, recurrence rate, mortality rate — were assessed in terms of odds ratio (OR) and 95% confidence intervals (CI). Calculations were performed using Review Manager (RevMan) software (Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014). A fixed-effect model was used when heterogeneity (I2) was <50%, whereas a random-effect model was used when it was >50%.
RESULTS
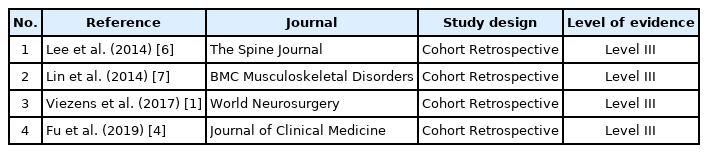
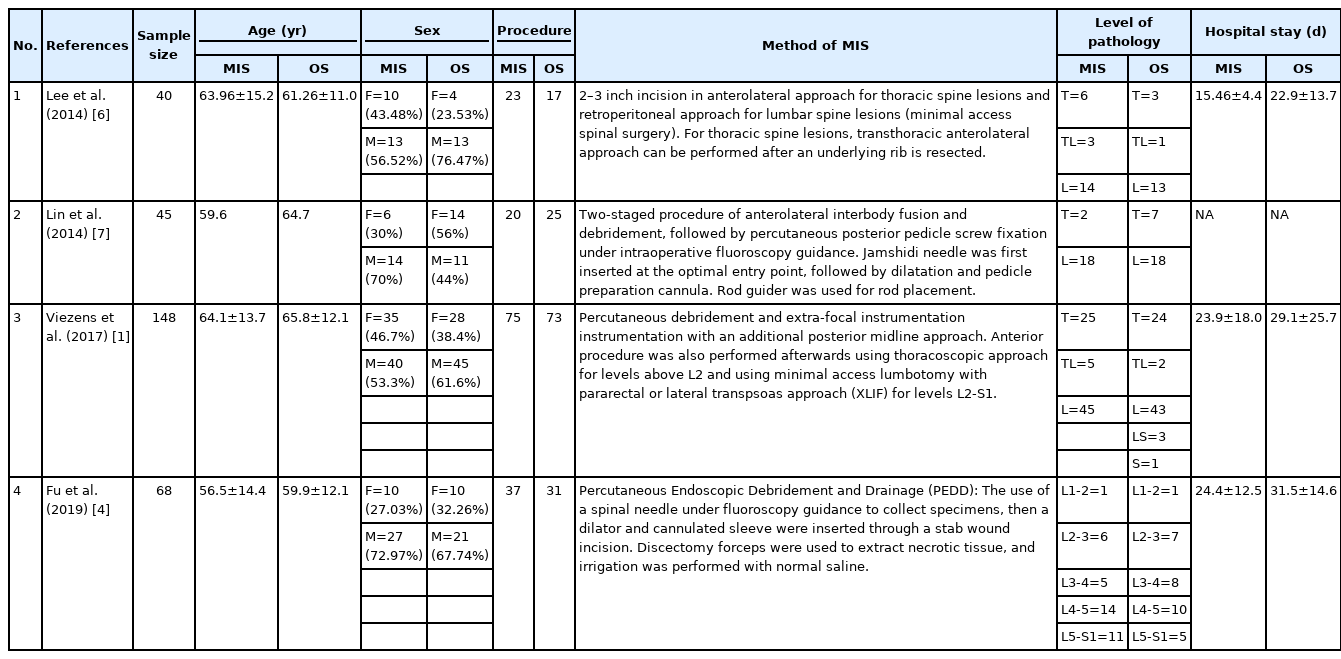
A total of four studies (301 patients) were included in the meta-analysis. All studies were of Level III evidence with cohort retrospective study design (Table 2). Critical appraisal of all studies based on the Joanna Briggs Institute Scoring System showed that none failed to meet more than four validity criteria (Figure 2).
The sample size for MIS was 155 patients, while for OS was 146 patients. In both groups, biopsy, decompression, and fusion were performed. The sample age ranges from the mean of 56.5–65.8 years old. Males were more commonly affected than females, in both MIS group (F:M=61:94) and OS group (F:M=56:90). Lumbar (L) was the most commonly affected region in MIS group (T:TL:L:LS=33:8:103:11) as well as OS group (T:TL:L:LS:S=34:3:100:8:1), followed by thoracal (T) region, lumbosacral (LS), thoracolumbar (TL), and sacral (S) region (Table 3). Pyogenic agent is a more common cause of spondylitis, with S. aureus as the most commonly found organism (MIS:OS=31:38), followed by S. epidermidis (MIS:OS=19:21). No pathogen growth was found in 24 cases in MIS and 22 cases in OS group (Table 4).
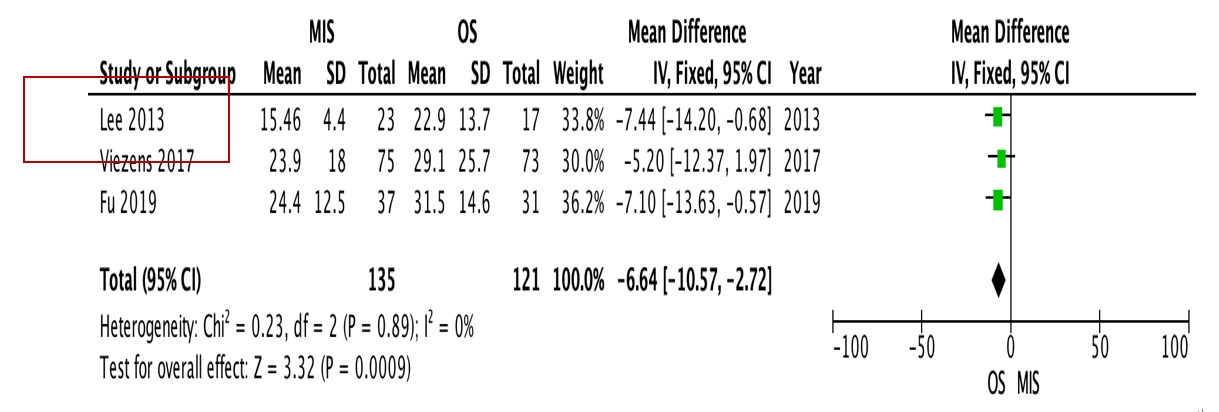
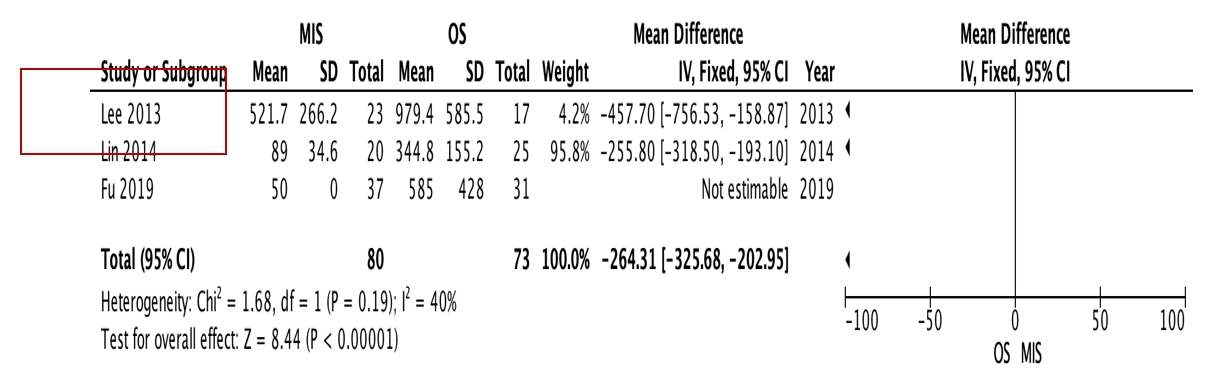
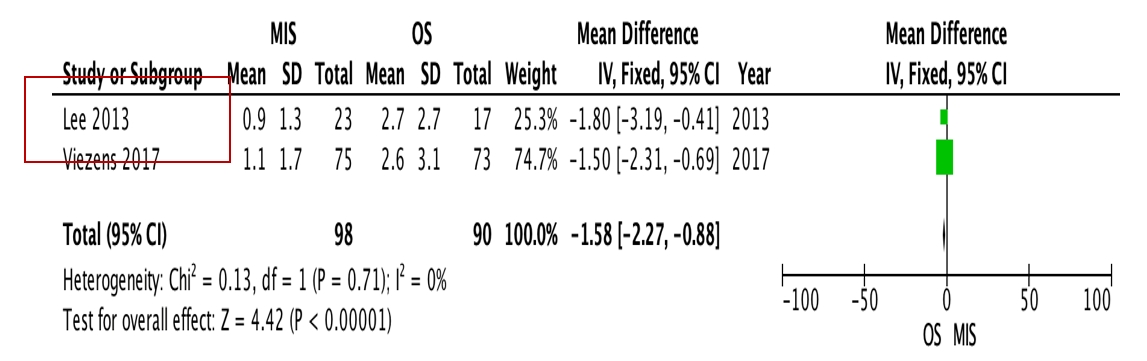
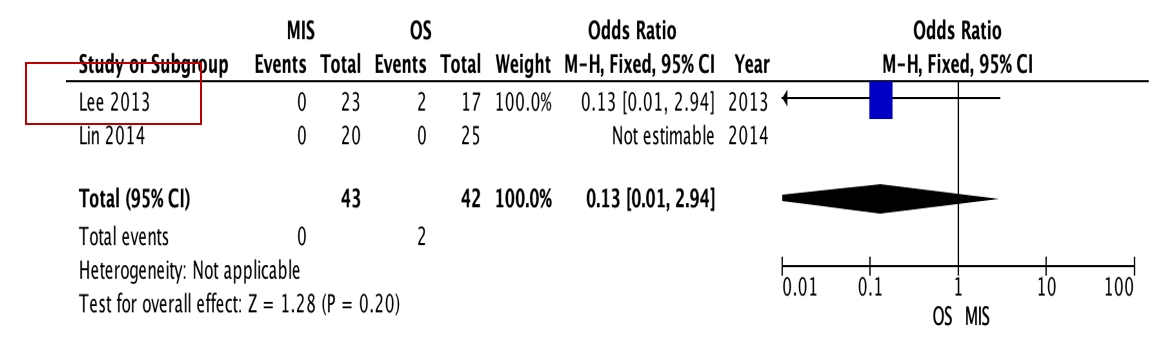
OS requires significantly longer hospital length of stay (p=0.0009, I2=0%, MD=–6.64, Figure 3) and higher blood loss (p<0.00001, I2=40%, MD=–264.31, Figure 4) as well as more postoperative blood transfusion (p<0.00001, I2=0%, MD=–1.58, Figure 5). Moreover, MIS has benefit in significantly shorter operation time (p<0.00001, I2= 46%, MD=–30.07, Figure 6) and less complication rate (p=0.0002, I2= 38%, MD=0.32, Figure 7). Some complications found are wound dehiscence, asymptomatic loosening, and transient paresthesia (Table 3). However, the two procedures do not differ significantly in terms of neurological improvement (p=0.37, I2=0%, MD=0.66, Figure 8), recurrence rate (p=0.02, MD=0.13, Figure 9), and mortality rate (p=0.28, I2=0%, MD=0.51, Figure 10). Postoperative pain after 7 days differs significantly between the two group (MIS:OS=2.8:3.5, p=0.03). Follow-up period ranges from 2–9 years (Table 5).
In terms of infection control, some different parameters were taken into account by each author. A study by Lee et al. (2014) [6] measured it from the amount of recurrent infection and death from sepsis, while Lin et al. (2014) [7] used recurrent infection, intraoperative complications (e.g. wound infection, screw loosening), and recurrent fever with elevated infection markers as parameters. Viezens et al. (2017) [1] and Fu et al. (2019) [4] used wound complications needing repeated debridement and screw loosening as signs of poor infection control. From our systematic review, the infection control exhibited by MIS seems to be comparable, if not better, than the OS.
DISCUSSION
Though nonoperative treatment was effective in 90% of uncomplicated cases, surgical intervention is needed in those who failed conservative therapy, or those with neurological impairments [4]. As a developing field in spine surgery, percutaneous endoscopic approach has been used more and more over the years, for the treatment of degenerative spine diseases as well as infectious spondylitis, especially for high-risk patients, elderly, and critical or immunocompromised patients [4,5].
The techniques for performing Minimally Invasive Debridement in infectious spondylitis varies in literatures. Fu et al. (2019) [4] utilized a spinal needle under fluoroscopy guidance to collect specimens, then a dilator and cannulated sleeve were inserted through a stab wound incision. Discectomy forceps were used to extract necrotic tissue, and irrigation was performed with normal saline. A drainage tube was inserted afterwards for several days postoperatively [4]. Another study by Viezens et al. (2017) [1] described percutaneous debridement and instrumentation with an additional posterior midline approach to achieve adequate laminotomy or laminectomy with abscess drainage and disc resection. Anterior procedure was also performed afterwards using thoracoscopic approach for levels above L2 and using minimal access lumbotomy with pararectal or lateral transpsoas approach (XLIF) for levels L2-S1 [1]. Lin et al. (2014) [7] in their study utilized two-staged procedure of anterolateral interbody fusion and debridement, followed by percutaneous posterior pedicle screw fixation under intraoperative fluoroscopy guidance. Jamshidi needle was first inserted at the optimal entry point, followed by dilatation and pedicle preparation cannula. Rod guider was used for rod placement [8]. On the other hand, a study by Lee et al. (2014) [6] described slightly longer incision of 2–3 inch in anterolateral approach for thoracic spine lesions and retroperitoneal approach for lumbar spine lesions. Chest tube or Hemovac drain was installed postoperatively [7].
Even though minimally invasive percutaneous approach procedure resulted in comparable fusion rate and good early functional recovery, MIS also generally has lower positive culture rate (58%–90%). This is possibly caused by the advantage of OS in obtaining direct access to the infected area for debridement and pus collection for further microbiology examination [6,9]. However, a study by Mao et al. (2019) [10] stated that despite its minimally invasive approach, PED can completely eliminate infected tissue, enhancing local blood flow, supporting antibiotic infiltration, and resulting in good clinical result.
In a study by Yang et al. (2007) [11], percutaneous spine procedure via endoscope was proven to be satisfactory, however prolonged pain and pre-existed anterior vertebral body damage caused postoperative immobilization for a long time, which could potentially lead to numerous complications. Thorough debridement and antibiotic therapy aid to eradicate the infection and control the progression of bony destruction, whereas the addition of instrumentation procedure is beneficial for early mobilization and reduction of kyphotic progression [3]. Technically, a study by Wu et al. (2020) [12] compares unilateral and bilateral percutaneous endoscopic debridement (PED) for lumbar spinal tuberculosis in 20 patients, concluded that unilateral PED is better than bilateral PED due to its shorter duration of surgery and comparable postoperative inflammatory markers, VAS, ODI, and complication rate.
Through the result of our study, MIS is proven to have significantly shorter operation time compared to OS, supported by the fact that MIS needs shorter duration for paraspinal muscle preparation and wound closure by the end of surgery. On the other hand, fluoroscopy time is longer in MIS, due to the increase of difficulty in visualizing spinal anatomical structure, especially when instrumentation is being performed. This counts as a disadvantage of MIS, as it prolongs the radiation exposure to the staff in operation room. However, these aspects are highly influenced by the learning curve and familiarity of the operator towards minimally invasive spine procedures [1].
In terms of complication, MIS displays lower rate compared to OS. Some complications mentioned for MIS are transient paresthesia or numbness, local infection, local kyphosis, and reoperation [7,12,13]. On the other hand, traditional open surgery has been associated with complications such as significant paraspinal soft tissue denervation, pleural effusion, diaphragm injury, and vascular injury (up to 15% with related mortality rate of 1%) [7,14]. To date, there have not been a lot of literatures mentioning surgical-related complications of PED for infectious spondylitis. A study by Fu et al. (2013) [15] stated that PED showed good efficacy as well as diagnostic value with no surgery-related complications in both uncomplicated and complicated infectious spondylodiscitis [16]. However, this matter might require further studies with larger amount of samples to draw more accurate conclusions, considering PED could be related to some neurological complications in the treatment of degenerative disc disease, such as postoperative dysesthesia. Sairyo et al. (2014) [17] in their study stated that the incidence of exiting nerve injury reached up to 8.9% in transforaminal approach. This is due to the direct injury by a cannula (supported by the fact that the patients were in anesthesia and unaware when the nerve was injured), or due to the prolonged intraoperative compression by cannula causing irritation of dorsal root ganglion (in which the leg dysesthesia typically occurs several days postoperatively) [15]. Other possible contributing factor to neurological problems in PED is the development of epidural or retroperitoneal hematoma, caused by possible injury to adjacent arteries [17,18]. Intracranial hypertension was also described in literatures, leading to headache, seizure, even death. This could happen due to the administration of contrast media into thecal sac, combined with dural tear, continuous infusion of fluid and medication. Therefore careful monitoring of perioperative symptoms and vital signs should always be performed, particularly the typical signs of seizures such as neck pain and increased intracranial or epidural pressure [15,17-19]. Some measures can be performed in order to minimalize these possible complications, such as using local anesthesia in conscious patients, careful monitoring of working channel position, and practicing the familiarity of local anatomy as viewed through endoscope [14,19,20]. Regardless all the benefits and complications of PED, traditional open surgery should still be considered in advanced stage infectious spondylitis, multilevel pathology, severe spinal instability, presence of abscess, and large bony defects, where further spinal reconstruction will be necessary [4].
To our knowledge, this is the first meta-analysis to objectively compare the efficacy between MIS and OS for infectious spondylitis. Another meta-analysis by Mao et al. (2019) [10] summarized the efficacy of percutaneous endoscopic debridement for spinal infection, however it was noncomparative study using one-arm analysis. This study has several limitations: (1) All four studies included into the analysis were of Level III evidence; (2) Due to the limited number of available studies, it was decided to include debridement procedure with and without instrumentation. Second-step procedure was also included into the analysis. This may bias our results, however we have ensured that the difference of baseline characteristics was not significant between control and intervention groups; (3) Incision length of the minimally invasive procedure was not exactly the same in all literatures. However, in all studies, the MIS procedures were all less invasive compared to traditional OS. This study also has several advantages: (1) To our knowledge, it is the first meta-analysis to objectively compare MIS and OS for Infectious Spondylitis; (2) The heterogeneity of all eight forest plots were <50%, reflecting the representativeness of studies included into the analysis (3) Outcomes were thoroughly assessed, in terms of several outcome measures, aiming to show the different dimensions of infectious spondylitis therapy. It is hoped that this study could serve as an influential bridge to future research with larger sample sizes, as well as a clinical guideline for choosing surgical therapeutic approach for patients with infectious spondylitis.
Notes
No potential conflict of interest relevant to this article.