Minimally Invasive Transforaminal Lumbar Interbody Fusion with Enhanced Recovery after Surgery (ERAS): Early Experience with Initial Consecutive Cases at a Spine Naïve Community Hospital
Article information
Abstract
Objective
The objective of this study was to examine a spine naïve community hospital’s ability to perform MITLIF safely and with speedy discharge via implementation of a minimally invasive spine surgery (MISS) program utilizing ERAS.
Methods
Single community hospital retrospective cohort analysis for initial consecutive MITLIF cases with unilateral pedicle screws performed by a single surgeon from October 2019 to March 2021. Minimum postoperative follow-up was one year. Narcotic use was assessed per the state prescription drug monitoring program. Surgery protocol included single paraspinal incision, non-expandable 18/22 mm tube, operating microscope, fluoroscopic guidance, EMG with SSEP monitoring and Enhanced Recovery After Surgery (ERAS) protocol.
Results
52 patients were included. Average OR time, and fluoroscopy time were 143±115 minutes, and 1.00±0.47 minutes, respectively. Patients were prescribed an average of 38±33 post-operative opioid doses for an average of 8±7 days. All patients on preoperative, chronic narcotics had no prescription changes, pre-op versus post-op, despite clinical improvement. Complications included one irrigation a(1.9%) nd debridement with retention of hardware for surgical site infection, and one revision(1.9%) for displaced hardware. Discharge data included 47 (90.4%), four (7.7%), and one patients (1.9%) discharged on POD1, POD2, and beyond POD2, respectively.
Conclusion
MITLIF can be safely and successfully performed at a spine naïve community hospital with excellent intraoperative metrics, a low complication rate, and speedy discharge. MITLIF performed well in multiple perioperative and postoperative variables compared to MISS techniques. Considerations for implementation of MITLIF in the community setting include special equipment, personnel training, surgeon experience, ERAS protocols and diligent patient/indication selection.
INTRODUCTION
Minimally invasive spine surgery (MISS) is an area of active research aimed at improving patient outcomes compared to open spine surgery techniques. MISS approaches these goals by utilizing minimal openings and natural surgical planes to reduce surgical blood loss, perioperative anesthetic and analgesic requirements, and to preserve posterior motion segments and paraspinal muscles [1]. Developments in imaging guidance technology and instrumentation led to the introduction of a minimally invasive transforaminal lumbar interbody fusion (MITLIF) technique in 2002 [2,3]. The goal of the MITLIF approach is to avoid the destructive impact of the extensive retraction and muscular dissection required for a traditional open transforaminal lumbar interbody fusion (TLIF) [4,5].
Currently, there are limited large, high-quality studies directly comparing MISS and traditional open approaches in lumbar fusion surgery. In early investigations, however, MITLIF has performed well compared to open TLIF in terms of reduced morbidity [6-8]. Despite these advantages, a recent meta-analysis comparing MITLIF to open TLIF found a higher revision rate and readmission rate in the MIS group [9]. These risks, combined with expensive specialized equipment, and the substantial MISS learning curve have contributed to some spine surgeons noting implementation obstacles in their practice [9-12]. Additionally, there is a paucity of research regarding the ability of performing MITLIF in the outpatient setting and a lack of research regarding applying Enhanced Recovery After Surgery (ERAS) techniques to spine surgery [13,14]. ERAS protocols are multimodal perioperative care strategies aimed at accelerating post-surgical recovery by optimizing nutrition, standardizing analgesic/anesthetic regimens, and encouraging early mobilization [15-18]. ERAS holds significant promise for amplifying the benefits of MISS by reducing the direct and indirect cost and patient burden of inpatient postoperative care, but further study is needed [19].
Our study addresses these gaps in the current MISS literature by detailing our institution’s implementation of both a MISS program and an associated ERAS protocol at a small, previously spine-naïve, community hospital. Our goal was to demonstrate that MITLIF, combined with an ERAS protocol, could be successfully implemented in the community setting without pre-existing perioperative spine infrastructure. The primary hypothesis is that MITLIF can be effectively performed in this environment and will result in excellent perioperative outcomes, early discharge, and a low complication profile. The secondary hypothesis is that MITLIF will perform well in comparison to other minimally invasive techniques performed in the same setting, which includes minimally invasive direct lateral interbody fusion (MIDLIF) and minimally invasive laminectomy with posterolateral fusion (non-interbody fusion, NIF).
MATERIALS AND METHODS
This retrospective chart review study was approved by the Institutional Review Board at the University of Pittsburgh.
1. Study Design
We conducted a retrospective chart review (level IV) of the initial consecutive cases performed between October 2019 to March 2021 by a single fellowship-trained orthopaedic spine surgeon. The setting for all cases was a university-affiliated community hospital with no in-house spine experience for over 10 years prior to this study. Inclusion criteria were patients that underwent a minimally invasive lumbar fusion procedure. These procedures included MITLIF, minimally invasive direct lateral interbody fusion (MIDLIF), or non-interbody fusion (NIF). All included patients were reviewed for demographic information including age, BMI, co-morbidities, indication for surgery, and gender. Perioperative information collected included total operative time, total radiation (fluoroscopy) time, surgery performed, complications, estimated blood loss (EBL), and any need for return to the operating room. Post-operative information collected included narcotics prescribed, length of hospital-stay, discharge disposition, and whether additional home health care was needed. Patient preoperative narcotic use was obtained via the Pennsylvania Drug Monitoring Program (PDMP). All patients included in this study had at least one year of follow-up available for review.
2. Treatment and Perioperative Protocol
Protocols included failure of nonoperative treatment modalities, which included over the counter medications, physical therapy, and interventional pain management. Surgical intervention included individualized patient-procedure matching with shared decision-making and pre-operative surgeon-guided education with counselling. Surgical technique included paraspinal/minimally invasive lateral lumbar surgery approaches. Surgical equipment utilized included a non-expandable 18/22 mm beveled and slotted tubular retractor ports, an operating microscope, and fluoroscopic guidance. Spinal monitoring was performed via EMG with SSEP. Spinal monitoring was used in all cases. Intraoperative and perioperative care followed an Enhanced Recovery After Surgery (ERAS) protocol. All cases included layered closure with barbed suture to reduce dead space, a local anesthetic consisting of bupivacaine administered along the wound bed in multiple small wheals, and a glue mesh waterproof dressing. Cases were performed without foley catheters, surgical drains, post-operative in-hospital imaging, or in-hospital dressing changes. For all patients, hospital medicine and occupational and physical therapy services were consulted on post-operative day (POD) 0. Laboratory bloodwork obtained on POD 1 included a complete blood count with a basic metabolic panel. Pain treatment included cold therapy, acetaminophen, methocarbamol, and narcotics as needed for breakthrough pain. Chronic pain prescriptions were maintained unchanged throughout patient hospitalization. Post-operative outpatient clinic follow-up visits were performed at 2 weeks, 6–8 weeks, and 16–24 weeks.
3. Statistical Analysis
The study power was set to 80% with an α=0.05. There were no missing values. The p-values were calculated from the likelihood ratio chi-square test for categorical variables including the subgroup difference in gender. Unpaired two-tailed t-tests were used to calculate p-values for continuous variables including average operating room (OR) time between subgroups. Linear regression was used for bivariate analysis with BMI.
RESULTS
1. Demographics
In total, 98 patients met criteria for inclusion. Patients were subdivided by surgical procedure performed. Group One consisted of 52 patients (53.1%) who had undergone MITLIF. Group Two consisted of 12 patients (12.2%) who had undergone MIDLIF. Group Three consisted of 34 patients (34.7%) who had undergone NIF.
Intergroup demographic comparisons included MITLIF versus NIF (Table 1) and MITLIF versus MIDLIF (Table 2). Patients in the MITLIF group were significantly younger than patients in the NIF group (66±12 years versus 73±10 years, p=0.005). Patients in the MITLIF group were similar in terms of gender distribution compared to the NIF group (28 females [53.8%] and 24 males [46.2%] versus 23 females [67.6%] and 11 males [32.4%], p=0.203). Patients in the MITLIF group had a significantly higher average BMI than patients in the NIF group (31.6±5.6 versus 28.9±5.1, p=0.027).
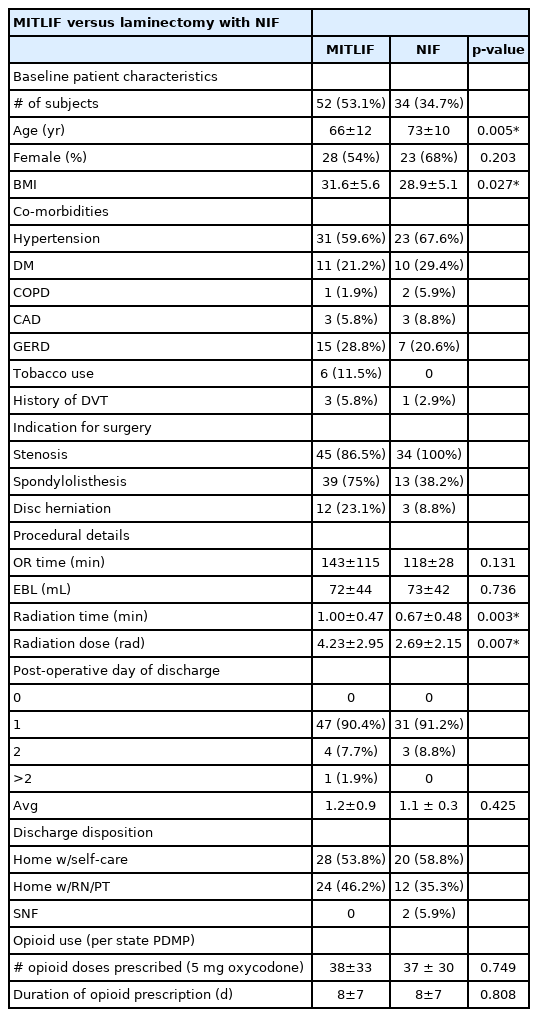
Intergroup comparisons between minimally invasive transforaminal interbody fusion (MITLIF) versus laminectomy with posterolateral fusion without interbody fusion (NIF)
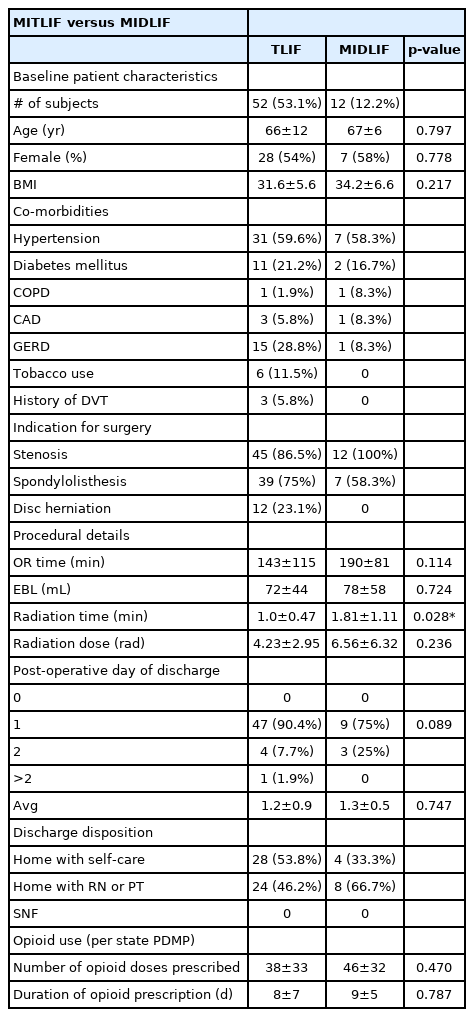
Intergroup comparisons between minimally invasive transforaminal interbody fusion (MITLIF) versus minimally invasive direct lateral interbody fusion (MIDLIF)
Patients in the MITLIF group were of similar age compared to patients in the MIDLIF group (66±12 years versus 67±6 years, p=0.797). Patients in the MITLIF group were similar in terms of gender distribution compared to the MIDLIF group (28 females [53.8%] and 24 males [46.2%] versus 7 females [53.8%] and 5 males [41.7%], p=0.778). Patients in the MITLIF group had a similar average BMI compared to patients in the MIDLIF group (31.6±5.6 versus 34.2±6.6, p=0.217).
2. Intraoperative Data
Intergroup intraoperative comparisons included MITLIF versus NIF (Table 1) and MITLIF versus MIDLIF (Table 2). Patients in the MITLIF group had similar OR time (minutes) compared to the NIF group (143±115 minutes versus 118±28 minutes, p=0.131). Patients in the MITLIF group had similar EBL (mL) compared to the NIF group (72±44 mL versus 73±42 mL, p=0.736). Patients in the MITLIF group had significantly longer radiation time (minutes) compared to the NIF group (1.0±0.47 minutes versus 0.67±0.48 minutes, p=0.003). Patients in the MITLIF group experienced higher radiation dose (rad) compared to the NIF group (4.23±2.95 minutes versus 2.69±2.15 minutes, p=0.007).
Patients in the MITLIF group had similar OR time (minutes) compared to the MIDLIF group (143±115 minutes versus 190±81 minutes, p=0.114). Patients in the MITLIF group had similar EBL (mL) compared to the MIDLIF group (72±44 mL versus 78±58 mL, p=0.724). Patients in the MITLIF group had significantly less radiation time (minutes) compared to the MIDLIF group (1.0±0.47 minutes versus 1.81±1.11 minutes, p=0.028). Patients in the MITLIF group experienced similar radiation dose (rad) compared to the MIDLIF group (4.23±2.95 minutes versus 6.56±6.32 minutes, p=0.236).
3. Postoperative Data
Intergroup postoperative comparisons included MITLIF versus NIF (Table 1), and MITLIF versus MIDLIF (Table 2). The number of patients discharged in the MITLIF versus NIF groups was similar on POD 0 (MITLIF: 0 patients versus NIF: 0 patients), on POD 1 (MITLIF: 47 patients [90.4%] versus NIF: 31 patients [91.2%]), and on POD 2 (MITLIF: 4 patients [7.7%] versus NIF: 3 patients [8.8%], p=0.87). The average day of discharge was similar between groups (MITLIF: POD 1.2±0.9 versus NIF: POD 1.1±0.3, p=0.43). Discharge disposition did not differ significantly between MITLIF and NIF patients (MITLIF: 28 patients [53.8%] home with self-care, 24 patients [46.2%] home with RN/PT, 0 patients SNF versus NIF: 20 patients [58.8%] home with self-care, 12 patients [35.3%] home with RN/PT, 2 patients [5.9%] SNF, p=0.66, 0.38, 0.15, respectively). MITLIF patients required similar number of opioid doses (5 mg oxycodone tablet) postoperatively compared to NIF patients (38±33 versus 37±30, p=0.749). MITLIF patients required similar duration of opioid prescription postoperatively compared to NIF patients (8±7 days versus 8±7 days, p=0.808).
The number of patients discharged in the MITLIF versus MIDLIF groups was similar on POD 0 (MITLIF: 0 patients versus MIDLIF: 0 patients), on POD 1 (MITLIF: 47 patients [90.4%] versus MIDLIF: 9 patients [75%]), and on POD 2 (MITLIF: 4 patients [7.7%] versus MIDLIF: 3 patients [25%], p=0.089). The average day of discharge was similar between groups (MITLIF: POD 1.2±0.9 versus MIDLIF: POD 1.3±0.5, p=0.75). Discharge disposition did not differ significantly between MITLIF and MIDLIF patients (MITLIF: 28 patients [53.8%] home with self-care, 24 patients [46.2%] home with RN/PT, 0 patients SNF versus MIDLIF: 4 patients [33.3%] home with self-care, 8 patients [66.7%] home with RN/PT, 0 patients SNF, p=0.34). MITLIF patients required similar number of opioid doses (5 mg oxycodone tablet) postoperatively compared to MIDLIF patients (38±33 versus 46±32, p=0.47). MITLIF patients required similar duration of opioid prescription postoperatively compared to MIDLIF patients (8±7 days versus 9±5 days, p=0.79).
Of note, chronic opioid dependence did not change despite clinical improvement. There were 8 patients in the MITLIF group and 2 patients in the MIDLIF group taking chronic opioids pre-operatively who had no difference in post-operative chronic opioid prescriptions.
4. Bivariate Comparisons
Amongst MITLIF patients, radiation dose was directly correlated with BMI (R2=0.1321). EBL (R2=0.0865), OR time (R2=0.0035), and opioid dose (R2=0.0069) were not correlated with BMI.
5. Complications
Complications included one MITLIF patient (1.9%) who underwent an irrigation and debridement (hardware retained) for surgical site infection.
DISCUSSION
Key Findings
The primary hypothesis of this study is that MITLIF, combined with ERAS protocols, can be effectively performed in the community setting and result in excellent perioperative outcomes, early discharge, and a low complication profile. Our data largely supported this hypothesis. Patients undergoing MITLIF had low EBL (72±44 mL), with 90.4% of patients discharged on POD 1, and 100% of patients discharged to home. The secondary hypothesis in this study is that MITLIF will perform well in comparison to other minimally invasive techniques performed in the same setting, which was also supported by our data. The use of MITLIF did not significantly increase EBL, OR time, length of hospital stay, opioid dose, or opioid duration compared to patients undergoing NIF or MIDLIF.
A recent study performed in a large university setting, which compared MITLIF to open TLIF, reported a median length of hospitalization of 3 and 4 days, respectively (p=0.006) [8]. Of the three randomized controlled trials comparing MITLIF to open TLIF, only one specifically examined postoperative hospital length of stay, and did not find a significant difference (6.4±2.5 days versus 8.7±2.1 days, p=0.087) [20-22]. Thus, our data indicate that implementation of MISS with ERAS protocols at a community, previously spine-naïve hospital can produce excellent discharge disposition and timing after MITLIF, even compared to large academic centers. There is limited data regarding outcomes after truly “outpatient” MITLIF, and in this study, no patients were discharged after MITLIF on POD 0 [13]. However, given that the ERAS protocol was not specifically catered to drive outpatient discharge, and with the retrospective nature of the current data, it is difficult to speculate from this study on the ability to perform truly outpatient MITLIF. Given the very short average length of hospital stay and excellent outcomes, it may be reasonable to perform a prospective analysis in this setting examining outpatient MITLIF.
Average opioid dose and duration required for postoperative pain control was low (38±33 doses of 5 mg oxycodone for 8±7 days). A recent work comparing MITLIF to open TLIF reported postoperative opioid usage of 167 and 255 morphine milligram equivalents, respectively [23]. The total average opioid dosage required after MITLIF in this study, when converted to similar units, was lower, at 57±49.5 milligram morphine equivalents. Good pain control and the very low EBL in this study are likely in part from a rigorous implementation of ERAS protocols. One of the only studies examining the use of ERAS in the setting of MITLIF reported a significantly decreased length of stay and EBL in patients undergoing MITLIF with ERAS compared to patients undergoing the same surgery with conventional postoperative protocols [19]. A recent meta-analysis examining studies reporting data on operative time in MITLIF versus open TLIF did not find a significant difference, but did note significant heterogeneity between the studies [24]. Amongst the studies reported in this meta-analysis, the mean operative time in the MITLIF groups ranged from 104±26 minutes to 389.7±57 minutes [25,26]. Our reported average operative time of 143±115 minutes compares well to these averages. Amongst the studies reported in this meta-analysis, the mean blood loss reported in the MITLIF groups ranged from 50.6±161 mL to 466.7±199.4 mL [25,27]. Our reported average EBL of 72±44 mL compares well to these averages. Thus, in terms of postoperative pain control, operative time and EBL, our data indicate that MITLIF can be safely and effectively performed in a small community hospital with no prior spine experience.
The wide range of data reported in the MITLIF regarding important intraoperative and perioperative variables can partially be attributed to a commonly cited shallow “learning curve” that exists in MISS in general, and especially for MITLIF [9,11,28-30]. The main reported impacts of this learning curve are on operative time and rate of complications. A recent study that mapped the MITLIF learning curve data to a negative exponential function reported that 90% of expert level operative time was achieved at case 39 [11]. Over this same time span, the complication rate dropped from 33% to 20.5% [11]. Additionally, due to a likely selection bias stemming from surgeons frequently electing to operate on simpler cases in the early stages of their experience with MISS, the impact of the learning curve in MISS is possibly underreported [3,31]. With these factors in mind, it is important to note that the operative surgeon in our study has been performing MISS for over 10 years. Thus, although the surgical center itself and the perioperative staff from our study were naïve to spine surgery, the operative surgeon is well past the learning curve reported for MISS in the literature.
The strengths of this study include that all cases were performed by a single surgeon at a single surgical center, which decreases confounding variables related to differing surgeon experience level, differing operative techniques, and differing perioperative staff. The weaknesses of this study include the significantly higher average age and significantly lower BMI of the NIF Group compared to the MITLIF Group, making direct comparisons between these two groups somewhat difficult. These differences were due to the retrospective nature of this analysis. A higher BMI, in particular, has been cited in the MISS literature as leading to increased radiation exposure. This was consistent with our findings when comparing the higher BMI MITLIF Group, which had significantly higher radiation time and dose, to the lower BMI NIF Group. However, while this body habitus difference did not appear to impact operative time, nor EBL, the differing age makes these findings difficult to fully interpret at this time.
CONCLUSION
This is the first data collected from a series of MITLIF cases performed after the establishment of an MISS program with ERAS perioperative care in a previously spine-naïve setting. It demonstrates the ability of a single surgeon, who is past the MISS learning curve, to achieve an excellent safety profile both in the intraoperative setting, upon discharge to home, and within a year of surgery, with excellent pain control after MITLIF with ERAS protocols.
Notes
Ethical statements
This retrospective chart review study was approved by the Institutional Review Board at the University of Pittsburgh.
Conflicts of interest
No potential conflict of interest relevant to this article.