Prodrome to Seizure in Transforaminal Endoscopic Surgery: A Series of 9 Cases
Article information
Abstract
Objective
Percutaneous transforaminal endoscopic lumbar discectomy (PTELD) is safe and effective. Perioperative or postoperative seizures are a rare complication that can be prevented by promptly identifying prodromal symptoms and signs. This study aimed to identify prodromal symptoms and risk factors of avoidable seizures in patients undergoing PTELD and to quantify irrigation fluid ingression into the epidural space on immediate postoperative magnetic resonance imaging (MRI).
Methods
This retrospective analysis included patients who underwent PTELD under local anesthesia from February 2018 to June 2022. Surgical records were reviewed to identify patients who developed prodromal symptoms, and immediate postoperative MRI was evaluated for radiological correlations.
Results
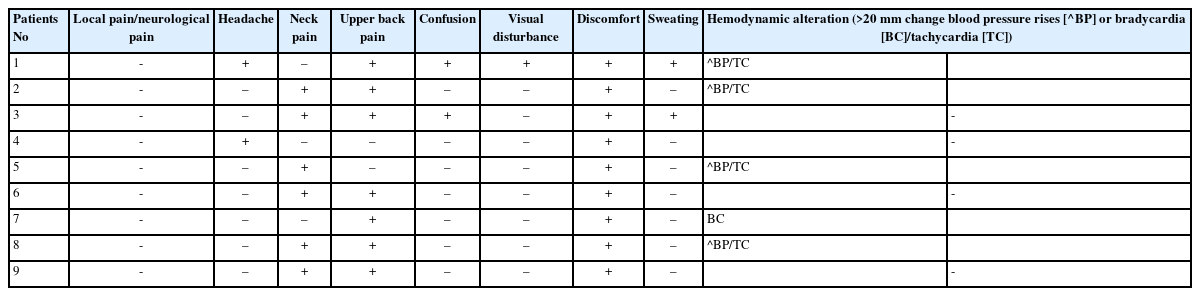
Nine patients developed prodromal symptoms of neck pain (n=6), upper dorsal pain (n=7), headache (n=2), confusion (n=2), visual disturbance (n=1) and hemodynamic alterations (n=4). No patients had seizures. Calcified lumbar disc herniation-associated posterior apophyseal ring fracture, central lumbar disc herniation, obesity, double-level surgery, use of an automated pump, and a large working channel endoscope were associated with an increased fluid flow rate for epidural work and duration of surgery. MRI showed significant epidural fluid collection cranial to the operative level, reaching the thoracolumbar junction, in patients with prodrome, suggesting increased intracranial pressure due to thecal sac compression.
Conclusion
Prodromal symptoms should be considered a red flag for avoidable seizures. The duration of surgery and infusion fluid flow rate are controllable risk factors during surgery. Risk factors should be kept in mind. The judicious use of automated pumps and larger channel working endoscopes is recommended.
INTRODUCTION
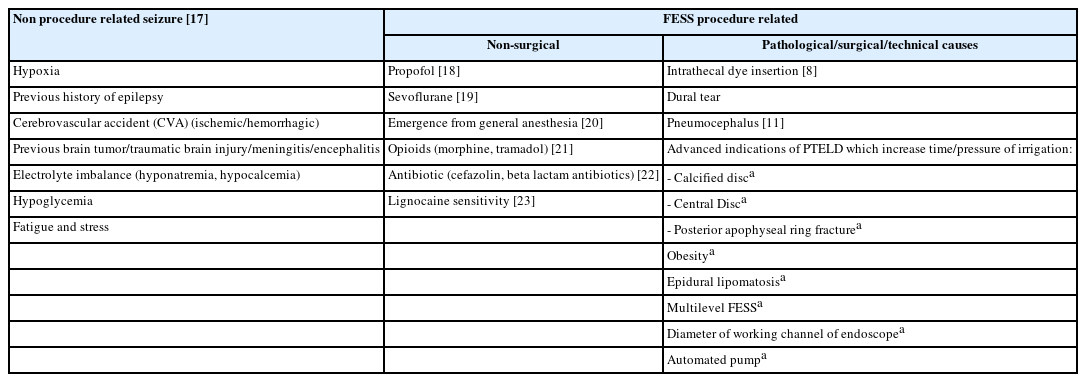
Percutaneous transforaminal endoscopic lumbar decompression (PTELD) is a minimally invasive technique for treatment of lumbar disc herniation (LDH) for discectomy and lumbar canal stenosis (LCS) decompression [1]. PTELD, PIELD (percutaneous interlaminar endoscopic lumbar decompression) are all full endoscopic spine surgeries (FESS) techniques and exponentially growing. The clinical results of FESS were identified as being equal to those of the microsurgical technique, adding great advantages as reduced post-operative pain, rapid rehabilitation, reduced anatomic trauma, low post-operative cost of care, facilitation of revision surgery [2,3]. PTELD is safe and effective, though with low rate of complications, such as neural and vascular structure injuries, dysesthesia, visceral injury, dural tears, infection, inadequate decompression of neural tissue and re-herniation [4]. Seizure in peri-operative and post-operative period of PTELD is an unexpected complication reported in previous literature [5-11]. In a study by Choi et al. [5] specific surgical factors like increased irrigation pressure and duration of surgery has been postulated as causative factors. The prodromal symptoms and sign for seizure episode in FESS are neck and upper dorsal pain, visual disturbances, headache, confusion and intra-operative hemodynamic alterations [5,9,12]. We primarily reinforce the hypothesis that the epidural fluid ingress cranio-caudal to operative level can give prodrome to seizures during PTELD surgery by raising the extra-thecal pressure (ETP/epidural pressure), intra-thecal pressure (ITP) and furthering to raised intracranial pressure (ICP) which may lead to full blown seizures. Secondarily, we identified and objectified factors in our cases which led to increase in irrigation pressure and duration of surgery. A literature search was done to find out the additional factors responsible to these fluid dynamics. Finally, the preventive methods are suggested.
MATERIALS AND METHODS
This is a retrospective analysis of all the patients who developed prodromal symptoms while undergoing PTELD under local anesthesia (LA) for LDH/LCS at our center from February 2018 to June 2022 (51 months). All the procedures were carried out by a single surgeon at our institute.
Prodromal symptoms were defined as occurrence of broad spectrum of pre-ictal symptoms that may be experienced during PTELD procedure. The symptoms can be neck pain, upper dorsal pain, headache, confusion, temporary visual disturbance, profuse sweating, confusion and or hemodynamic disturbance. A sudden rise or fall in mean arterial pressure (MAP)>20 mmHg, sudden alteration in pulse rate (PR)>20 from preceding baseline were considered a significant hemodynamic alteration.
The demographic features were analyzed from medical, surgical, and imaging records to find out a plausible explanation. Age, sex, any pre-existing aggravating condition for instigating episode of seizure if any, Body mass index (BMI), level of surgery, number of surgical levels, grade of lumbar disc herniation as per MSU classification [13], type of offending pathology and associated conditions projecting into the spinal canal. The definitions were: posterior apophyseal ring fracture (PARF); separation of the bony fragments at the posterior rim of the lumbar vertebral endplate (superior or inferior). Calcified lumbar disc herniation (CaLDH); calcification or hardening of the annulus in herniated part of LDH or associated with calcified end plate spurs (Figure 1). LDH is nucleus pulposus herniation with or without end plate cartilage dislocation and annular avulsion/tear. LCS is ventral or lateral recess stenosis [14].
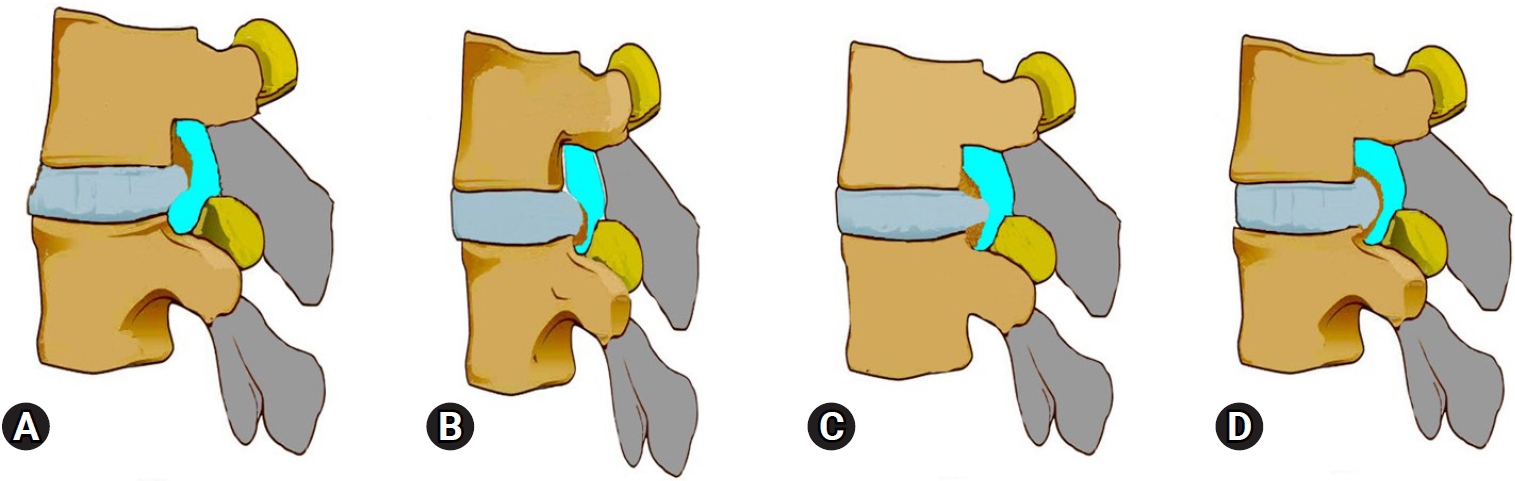
Illustration of the calcified disc component of ventral stenosis as noted with respect to PTELD. (A) Only an upper vertebral end plate spur contributing to stenosis with lumbar disc herniation (LDH). (B) Only a lower vertebral end plate spur contributing to stenosis with LDH. (C) Both end plate spurs contributing to stenosis with LDH. (D) Only annulus hardening or partial calcification with LDH.
An independent anesthesiologist was present during the surgical procedure. The surgery was done in LA and conscious sedation in prone position. Intramuscular midazolam (0.05 mg/kg) and diclofenac were given within one hour of surgery. Half a dose of titrated infusion of dexmedetomidine (0.5–1 μg/kg) was given slowly with an intravenous dose of 1 μg/kg of fentanyl bolus ten minutes before the surgery. Additional doses as needed were given.
A transforaminal approach through Kambin’s triangle was used. Fluoroscopy was used for guidance of entry point. The site of annular puncture is medial pedicular line in AP view and posterior vertebral line in lateral view. The skin entry point is around 9–14 cm from the midline and at angle of 20°–30° from frontal plane of body depending on level of surgery performed. The intended entry tract is infiltrated with 1% lidocaine plus bupivacaine in 1:1 ratio. A 16-inch 18-gauge needle is inserted to site of annular puncture in Kambin’s triangle with continuous patient’s feedback. Trajectory can be changed by bevel of the needle, and cranio-caudal or dorso-ventral lift of the stylet hub of needle before steering it further. Site of annulus puncture is further infiltrated with 1.5–6 mL of local anesthetic mixture. Constant communication with the patient eases out anxiety.
Through a 7 mm incision, threading of tapered dilating trocar is done over a guidewire. The beveled working cannula was railroaded, and endoscope was introduced (Maxmore system-Germany/Richard wolf system-Germany). The removal of the offending compression was done with straight and articulated instruments to cut, tease, grab, deliver the culprit fragment. LDH excision was done along with optimum compressive ventral stenosis removal. The techniques employed depended upon the pathology and were standard basic techniques of outside in (OI) or inside out (IO) or FEE (flat epidural entry), with modifications as needed for the cases at author’s institute.
Inside out (IO) was the workhorse approach: An approach at a 15° to 20° angle was taken. The disc was pierced, and the sub annular disc was removed before cutting the annular anchorage and working in epidural space.
Under endoscopic vision a burr was used to perform a foraminoplasty when there was a technical requirement to reach more dorsal. Endoscopic drill system (Nouvag system, USA) was used during the procedure. Offending or compressive structure is removed completely. End points of decompression were as many possible of the suggested endpoints of decompression in author’s previous article either direct or indirect were targeted [15]. The direct signs of end point of decompression were magnetic resonance imaging (MRI) matched retrieval of fragment, fresh gush of epidural bleed, neural fall back and complete visualization of roots, dural sac, strong dural pulsation, dural flutter on cough impulse. The indirect signs of end point were subsidence of pain, intra-operative negative straight leg raise test, annular flap mobility, smooth sweeping of discal floor etc.
Skin incision was closed, and all patients are mobilized as per their tolerance and limb power. Patients were then advised to undertake passive/active physiotherapy.
The technical difficulty faced during the PTELD due to targeted disease pathology or patient factor, or any special technical accessory/modification used in the case were noted. When prodrome occurred then, the PTELD was halted, irrigation fluid was stopped, ventilation with O2 mask given, vital monitoring done, patient counselled and stabilized to comfort. After the prodrome has passed, the procedure was again continued till completion.
The duration of surgery (minutes) was noted from positioning of patient to end point of decompression, duration of irrigation of fluid (minute), irrigation flow method (gravitational/automated pump), frequency of usage of intermittent manual pulsed pressure (IMP) and the ingressed fluid volume in mL were noted. Gravity method used at our institute. It constituted of non-latex irrigation tubing connected to 3 L of normal saline suspended at a height of 1.5 m from the height of table to maintained probably a pressure of 74–110 mm H2O (Figure 2A, B). IMP was used whenever higher pressure was needed for hemostasis for clear vision. The valved hand pump inflator connected to 3 L of saline bottle was used to give IMP. This was done with an ETO sterilized custom-made valved inflation bulb (Figure 2C) which was introduced into the saline bottle and manually inflated alike a manual sphygmomanometer bulb. Each pulse by experience arbitrarily had been fixed at five manual compressions of inflation bulb, which suffices to take care of a FESS epidural bleed. In a usual surgery it’s been noted that for PTELD in a simple LDH, less than 5 pulse (5 compression×5 times) are needed. The need for pulses in our experience of 14 years of performing PTELD, increases with complexity of procedure, need for epidural work, bony stenosis, and duration of surgery. The number of IMP pulse that was used were objectively categorized as less than less than <5, 6–10, 11–15, 16–20, and >20.
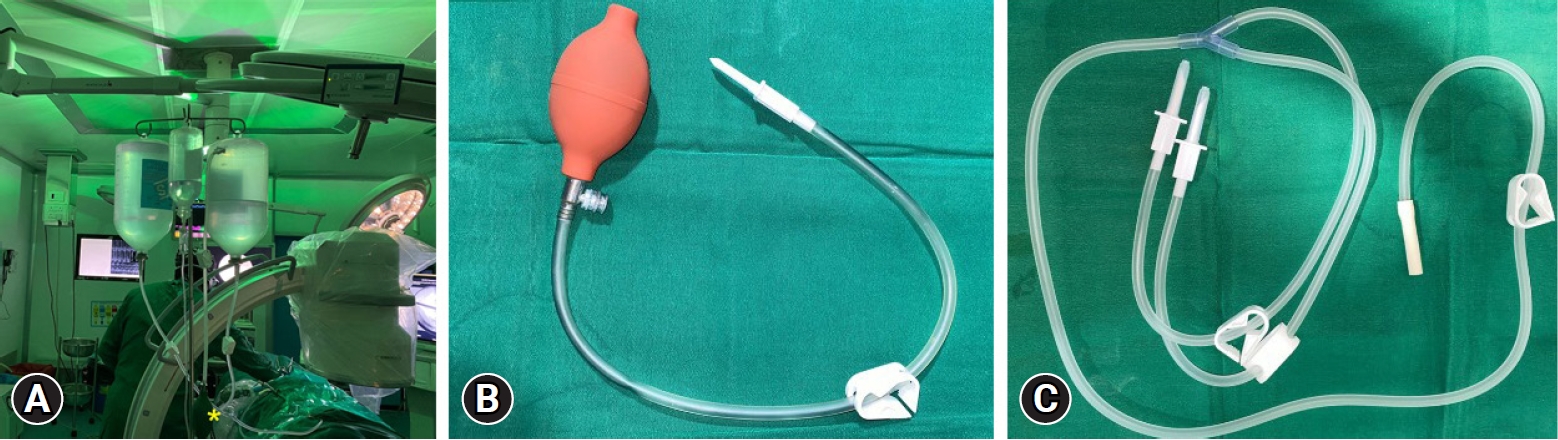
(A) Hydrodynamic irrigation pressure setup. (B) A TURP double-lumen tube, one working at a time connected to two 3-L saline bottles hanging by a drip stand around 1.5 m from the table height. (C) Sterile plunger tubing with a manual pressure pump (a valved bulb). The trocar of the plunger is plunged into the air column part of inverted saline bottles, and intermittent manual pressure pulses by the bulb (yellow asterisk*) are given when any obscurity of endoscopic visualization due to bleeding occurs.
As a protocol, at our institute immediate post-operative MRI T2 sagittal and axial sequences at the operated levels are done within two hours in all patients for documentation and for conformity of decompression in all cases of PTELD. Additionally, in all the patient who had prodromal symptoms underwent the MRI up to section of lower dorsal spine.
MRI was reported by a spine radiologist with more than 10 years of experience in only spinal disorders reporting. The reporting was categorized as adequate/inadequate decompression and presence of post-operative atypical findings. Atypical finding was the presence of fluid in epidural space more than two segment level. The presence of fluid ingress in epidural space was further jotted as cranio-caudal to index operative level. The number of segments above and below encroached by fluid was noted. Furthermore, it was documented whether it was anterior or posterior or circumferential. Myeloblock or any compression on dural sac other than index surgical compression was also noted above the operated level.
The follow up period, evaluation of functional outcomes was done using VAS score in leg and back, the patient satisfaction index [16], Macnab outcome score were noted with any other complications.
RESULTS
During the study time period of 51 months, 290 patients were operated for PTELD and 9 patients developed prodromal symptoms. No patient developed post-prodromal seizure sequalae. Detailed description of patient demographics who developed prodromal symptom during PTELD is tabulated in Table 1.
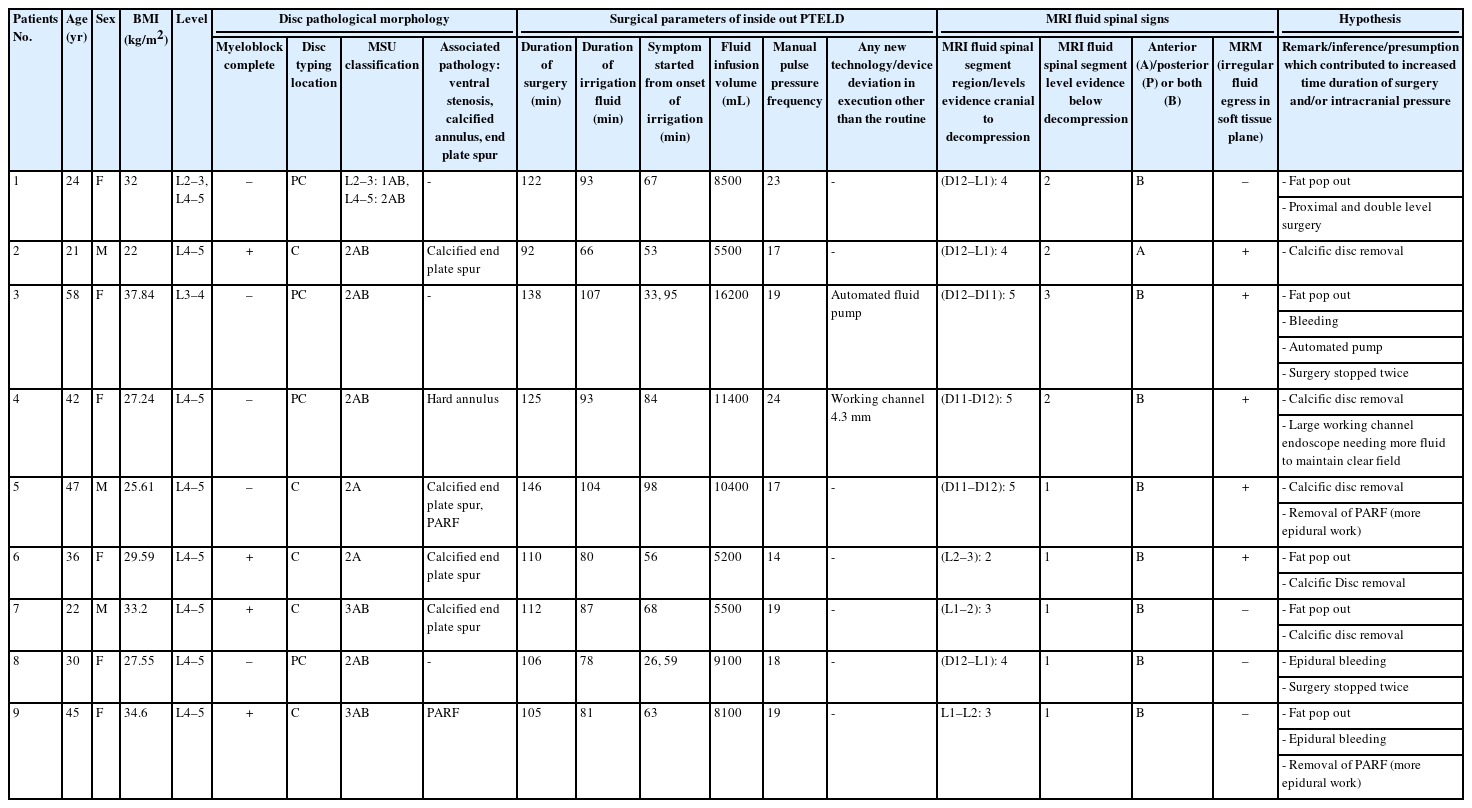
Detailed description of the demographics, radiological findings, and surgical records of patients in whom prodromal symptoms developed during PTELD
There were 3 male and 6 female patients (average age 36.1±12.85 [21–58] years). Four patients were obese and 4 patients were overweight as per BMI. The most common level of LDH in our study was L4–5 (n=8). One patient had double level surgery at L2–3 and L4–5. As per MSU classification: 2AB (n=5), 3AB (n=2), 2A (n=2) and 1AB (n=1) out of total 10 LDH levels. Four patients had complete myelographic block. Five patients had calcification (CaLDH), which was annulus calcification in 1 and endplate spurs in 4 patients. PARF was the pathology in 2 patients.
Surgery was done using the basic techniques of IO in all cases under LA. In patients with CaLDH, the IO technique needed additional technique modifications of calcified ventral decompression for removal of the hardened tissue. Additionally, burred foraminotomy was needed in one patient for approaching dorsal canal, medialization and improving the reach in epidural space.
The prodromal symptoms started at 60.89±22.59 (26–98) minutes from onset of irrigation of saline, towards the end stage of surgery in majority (n=7) of patients. All the 9 patients had started to complain of non-painful discomfort omit at start of prodromal symptoms. Five patients had hemodynamic disturbance in which 4 patients had sudden shoot up in blood pressure and tachycardia and one developed bradycardia (Table 2). Procedure was halted till prodrome passed by and 3 patients were given prophylactic intravenous levetiracetam. Remaining procedure could be executed afterwards. In two patients, procedure had to halted twice due to prodromal symptoms.
The total fluid volume of saline infused was 7.96±2.53 (5.2–16.2) L. Multiple IMP was needed during surgery to maintain unobscured vision in all the patients. Epidural fat pop out hindered the endoscopic vision in 5 cases all were obese. These patients required more IMP to keep the endoscopic vision.
Technical novice of automatic pump was experimented before a proposed buyout for the first time by author in one patient. The irrigation fluid flow rate was started and maintained at 150 mL/min. Endoscope of a larger diameter of 4.3 mm was used in another one case instead of routine 3.7 mm used by author otherwise always for another proposed buyout at the institute.
Post-operatively, adequate decompression was confirmed on MRI for index pathology in all patients with improved immediate patient outcome measures. Irrigation fluid collection in epidural space in cases with prodromal symptom was an average of 3.8 segment above and 1.5 level below the operated level causing thecal sac compression. The fluid collected circumferentially (both anterior and posterior epidural space) in 8 cases and anterior epidural space only in 1 case. The irrigation fluid extravasated extra canalicular into adjacent soft tissue in irregular patten, which vas visible in magnetic resonance (MR) myelogram in 5 cases. MR myelogram showed variable segment myeloblock was present in all patients post-operatively without any symptoms (Figure 3, 4).
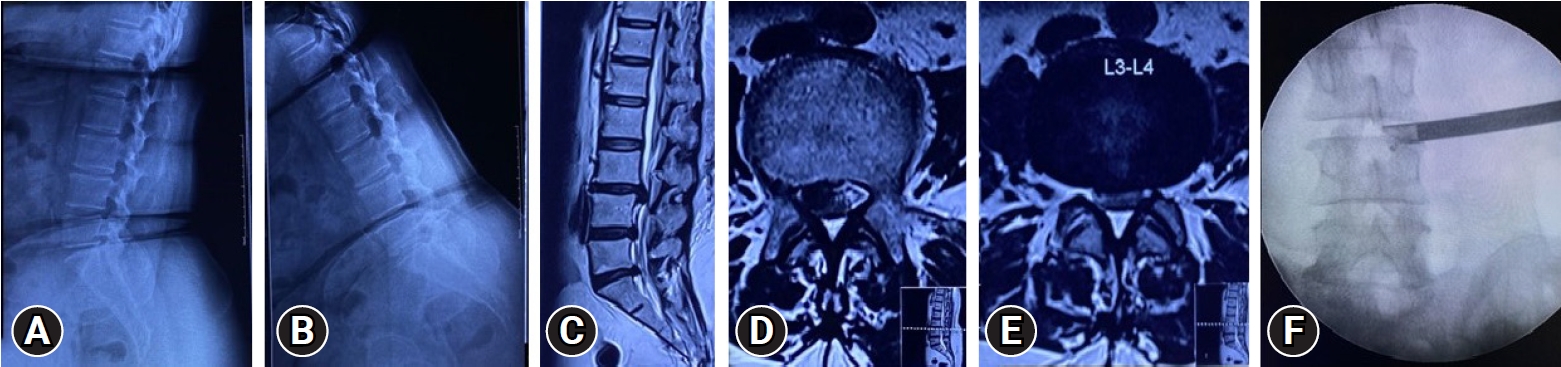
Case No. 3. A 58-year-old woman in whom conservative treatment had failed, with a 9-month history of unilateral right radiculopathy with disability. (A, B) No instability on dynamic radiographs. (C–E) T2-weighted sagittal and axial magnetic resonance imaging sections showing L3–4 right MSU classification 2AB discs. More fat than usual is present in the epidural space. (F) The “inside out” approach used in surgery, with transforaminal endoscopic discectomy decompression. The image shows the perioperative position. Perioperatively, the fat popping out obstructed the endoscopic visualization, requiring more time and fluid for decompression.
Case No. 3. Immediate 2-hour postoperative magnetic resonance imaging (MRI) showing: (A) A magnetic resonance myelogram showing extra spinal fluid and myeloblock. (B) T2-weighted mid-sagittal MRI showing adequate decompression, but a fluid intensity strip around the dura in the ventral, dorsal, and cranio-caudal space. Also noted is a small air bubble in the dorsal epidural space at upper end of L3. (C) Proximal to the L3 mid-vertebral body, an axial T2-weighted image shows the clumping of roots, and fluid (red asterisk*) with the median posterior longitudinal ligament. (D) Proximal to the L1 mid-vertebral body, an axial T2-weighted image shows the clumping of roots. Dorsal to the dura, it is appreciable that the fluid (red asterisk*) is ventral to the posterior epidural fat. (E) At 6 weeks of follow-up, T2-weighted sagittal MRI shows the disappearance of fluid (as compared to Figure 5B),the reappearance of fat ventrally in the upper epidural space (as compared to Figure 4C) and consolidating annular healing without compression.
The follow up period was ranging from 3–25 (9.65±9.32) months. The evaluation of functional outcomes were done using VAS score in leg and back. The VAS score of leg improved from 8.2±1.3 pre-operatively to 0.44±0.52 at final follow up. The VAS score of back improved from 3±1.18 pre-operatively to 1.11±0.33 at final follow up. The patient satisfaction index was 1 in all patients. The clinical success of procedure measured Macnab outcome as excellent in 7 patient and good in 2 patients. No other complications were noted.
DISCUSSION
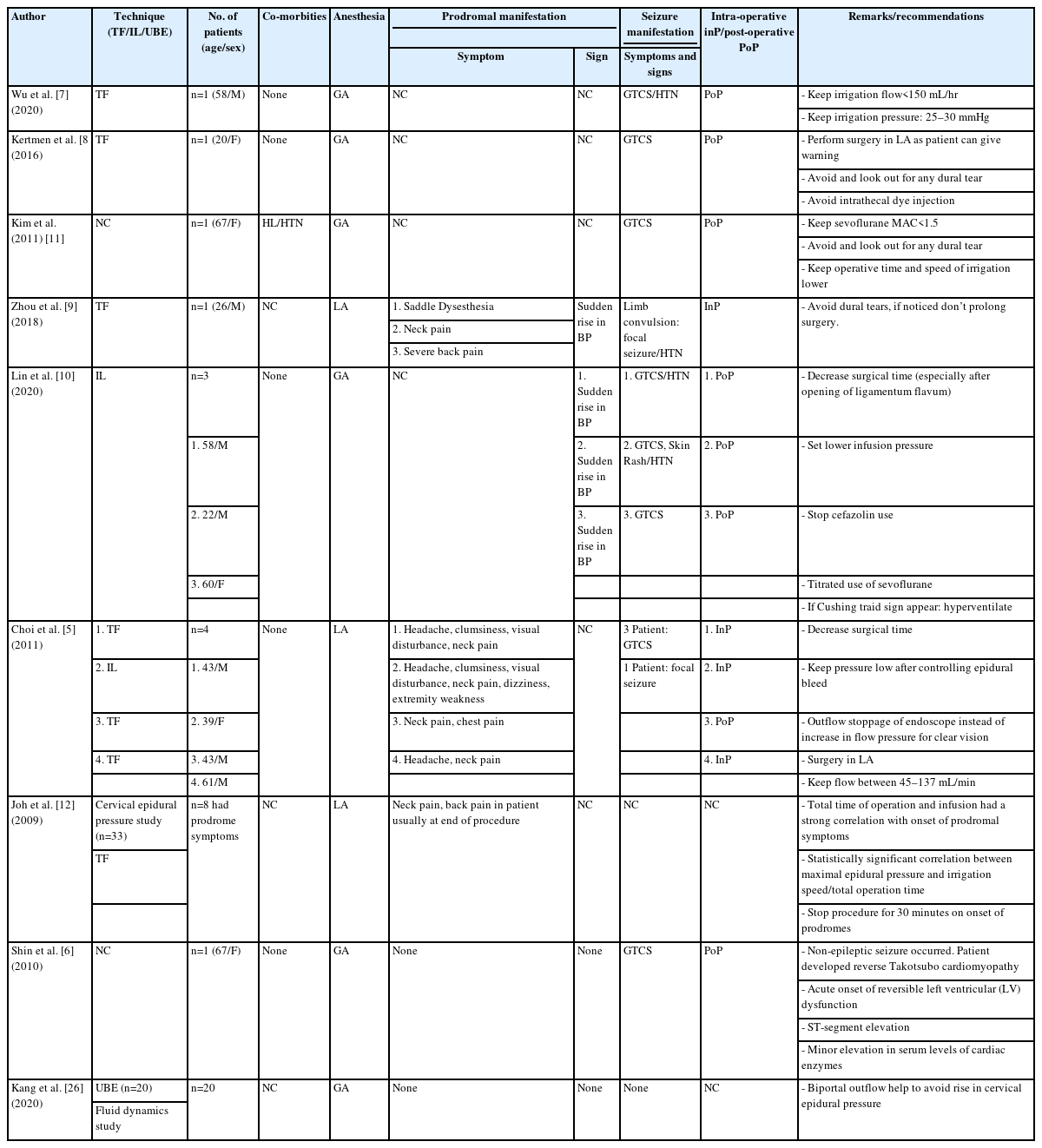
The prodrome of an already manifested seizure has been described in general medical literature [5,9,17]. These prodromes are neck pain, back pain headache, visual disturbances, dizziness, and constriction feeling in saddle area and chest pain [5,9,12]. Identification of prodromes could be a red flag sign should be the focus especially if done under LA, as carrying out the PTELD under general anesthesia (GA) can mask the early symptoms of elevated ICP leading to serious and devastating complication. LA also allows communication between the patient and surgeon which could alert regarding the impending seizures [5]. Its logical to infer that when FESS is done under GA, chances of manifestation become less as patient is on medications which have suppressive effect and increase the threshold of seizures.
Although the definite cause of seizure in PTELD/PIELD surgeries remains uncertain, many postulates have been made in literature relating to patient characteristics, intra-operative and surgical factors. There are many causal possibilities of seizures in FESS (PTELD) specifically or in general and are tabulated (Table 3) [8,11,17-23].
Increasing dose of sevoflurane is known to increase the epileptiform changes in electroencephalogram (EEG) and it is advised to keep ha minimum alveolar concentration below 1.5 to reduce chances of any seizure activity. Rapid decline in sevoflurane concentration, sudden CNS excitation, and hypercarbia while extubating and re-emergence from anesthesia, can be a potential risk factors for seizure [19,24].
Generalized tonic clonic (GTC) seizure in PTELD for LDH in GA has been reported due to probable causative factor of pneumocephalus due to dural tear, prolonged saline irrigation and sevoflurane/nitrous oxide (N2O) anesthesia. Low blood gas partition coefficient of N2O contribute in raising ICP by expanding the volume of the pneumocephalus [11]. Inadvertent administration of the non‑ionic contrast media (iohexol) into the thecal sac in PTELD has been reported [11]. Cephalosporin has also been reported as potential epileptogenic because of the antagonism of the GABA receptor, durotomy or allergy [10].
The epidural space is a potential intra-canalicular space that extends throughout the spine. Continuous irrigation with normal saline is required in PTELD to maintain a clear endoscopic visual field for proper surgical execution. The hypotheses by Choi et al. [5] was that the ETP due to continuous infusion of saline into the epidural space could compress the thecal sac in cephalad direction. This could increase the ITP and that in turn raises ICP (Figure 5). The maximal cervical epidural pressure in those with neck pain has been significantly higher than those without neck pain suggesting, epidural pressure can affect the ICP [5,12]. It was also related to the time of surgery. It’s been seconded in multiple other studies. [7,10,25,26]. So, continuous fluid infusion rate/pressure and duration of surgery are the two postulates of increase ETP in a PTELD.
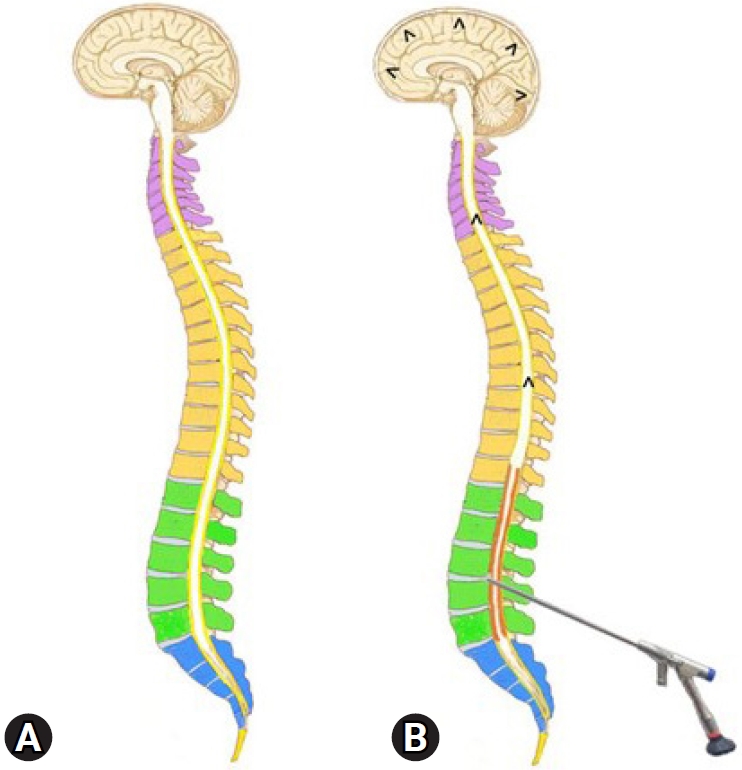
(A) Illustration showing the epidural space as a potential space that extends throughout the spine and brain. (B) The increased epidural pressure due to a long-duration saline infusion would compress the thecal sac and expand the potential space with irrigation saline (red), squeezing out the cerebrospinal fluid cranially and precipitating seizure.
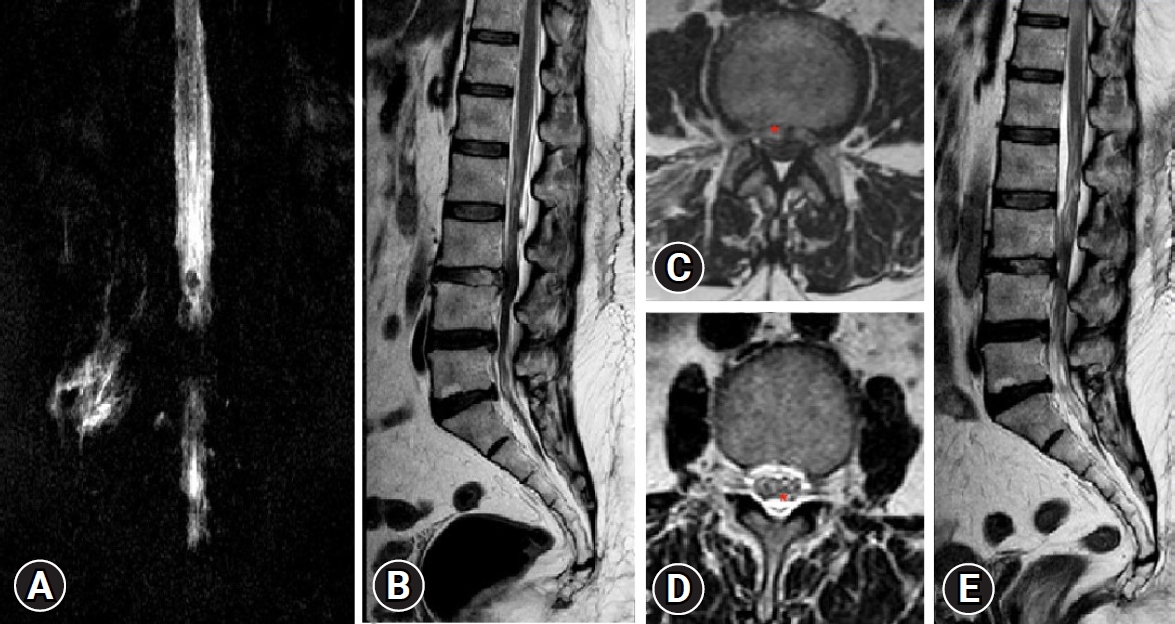
Higuchi et al. [27] objectified on MRI and found that the thecal compression was volume dependent. In literature, people with no intracranial pathology, epidural pressure (EP) and intracranial pressure (ICP) has been shown to exhibit a linear co-relation [25,27,28]. In this study, we noted presence of fluid collection around thecal sac on T2W sagittal and axial section of immediate post-operative MRI in patients who developed prodromes (Figure 6). The possibility that increased ICP occurred was strongly considered.
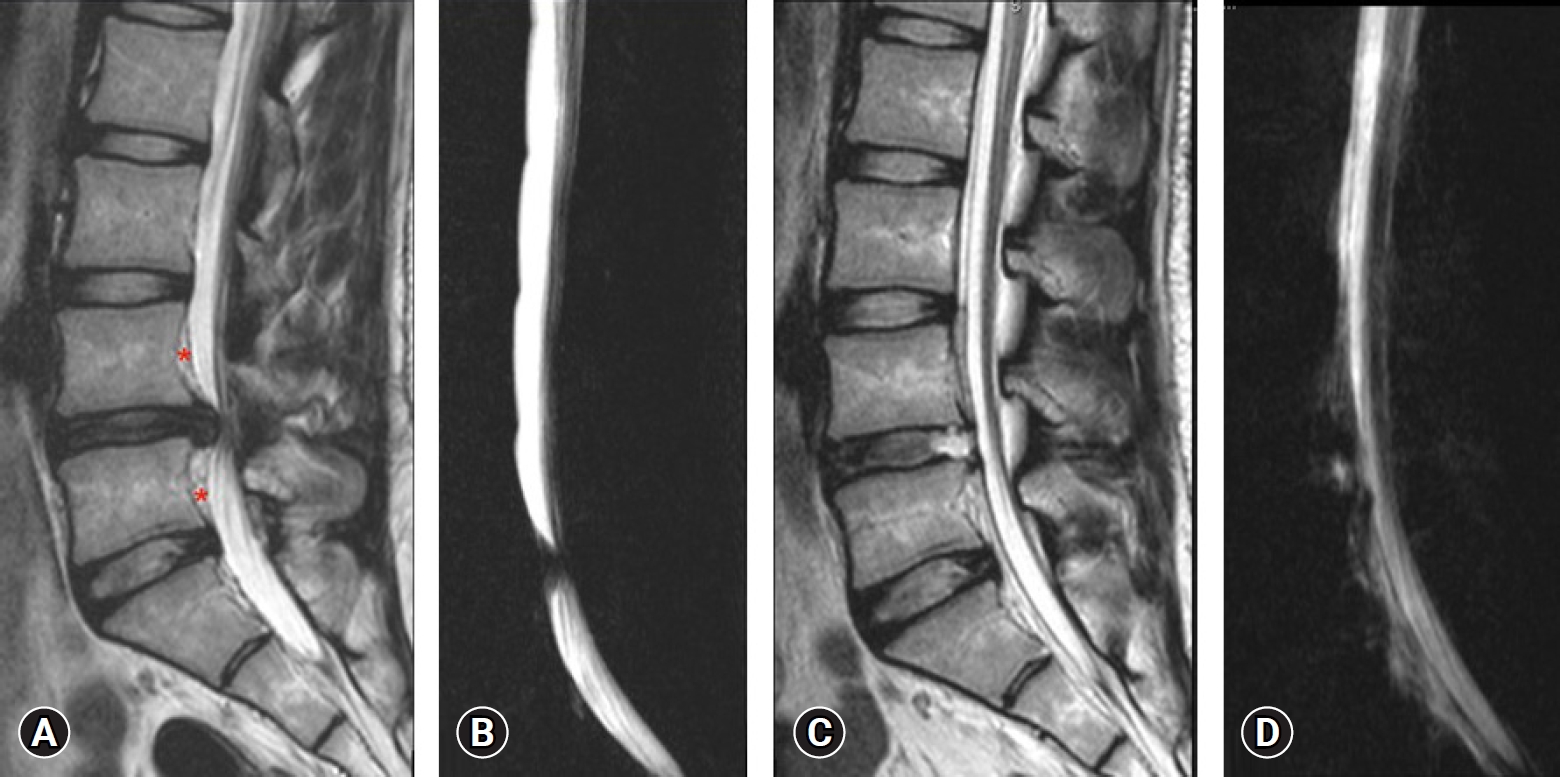
Case No. 2. (A) T2-weighted sagittal magnetic resonance imaging (MRI) showing central disc herniation. Chronicity is evident by the fat deposits (red asterisk*), posterior to the vertebral body of L4 and L5 and ventral to the posterior longitudinal ligament. It is elevated by both end plate spurs. (B) Lateral magnetic resonance myelogram showing complete myeloblock. (C) Postoperative T2-weighted sagittal MRI showing complete decompression. A thin strip of fluid three segments above and below, ventrally only. (D) Postoperative lateral magnetic resonance myelogram showing extra spinal fluid and an indenting anterior compressive fluid strip making the margins indistinct and wavy.
The natural and physical pathway by which fluid egress/outflow regulates the ETP in FESS The fluid egress is through separate channel or working channel of the uniportal endoscope in FESS. Leakage of fluid along dural sleeves of spinal nerves and foraminal spaces also occurs and is related to the size of the intervertebral foramen, the density of the fatty tissues around it, any adhesions, and foraminal stenosis [29]. Lymphatic drainage in epidural space is another possibility [30]. Additionally, the rise in ICP will be dependent on intra-cranial compliance of the patient and by the amount of intra-cranially absorbed CSF [27,28].
The spectrum of indications for PTELD are directly related to the surgeon’s experience [14,31]. The surgical duration increase occurs with complex pathological subtypes dealt with PTELD like CaLDH, CLDH, obesity, multiple level surgery, upper lumbar PTELD and PRAF. CaLDH was considered an absolute contraindication in a demi decade old literature. This was due to difficulty and increased failures [31].
Lumbar posterior apophyseal ring fracture (PARF) is characterized by the separation of the bony fragments at the posterior rim of the lumbar vertebral endplate. Conventionally open surgery has been a standard method of care for PARF over the past 3 decades. Successful removal of PARF using FESS has been described and is still in nascent stages [32]. In a study by Yuan et al. [33] the mean operation time in the LDH with PARF was significantly more than LDH without PARF using PTELD (105.4 min vs 83.9 min) (p=0.001) and surgical complications of dural tears (6.3%) were more common in the PARF group (p=0.025). PARF being a more technically demanding indication, required more of epidural work during surgery.
Calcified disc herniation (CaLDH) is a subtype LDH with calcification of herniated fragments [14]. The chronicity of herniation leads to calcification of annulus, or the end plate spurs contributing to ventral stenosis (Figure 1). PTELD gives an advantage of direct access to ventral pathology, minimal retraction of root, a more in-plane dissection, avoiding retraction of neural tissue as against in posterior approach. A CaLDH is still difficult to treat using PTELD due to severe adhesions and more epidural work needed [34] Yuan et al. [35] reported a reasonable outcome follow up of PTELD in CaLDH in 50 patients and 52 patients with uncalcified LDH with a statistically significant operative time duration increase with report of neck pain incidence.
Spinal epidural lipomatosis (SEL) is a condition of overgrowth of adipose tissue within spinal canal which can be either asymptomatic or can present with symptoms of canal or foraminal stenosis [36]. Obesity is thought to be the most common cause of SEL. Incidental durotomies are also reported more up to 9.4% in obese patients. Though exact details of technical difficulties were not mentioned but anatomical obscurity is reported [37]. While doing PTELD in patients with more epidural fat, the fat pops into the operating field and obscures visibility and bleeding at times and may make the end point of decompression fallacious [15]. So, to keep the field clear, more irrigation pressure is needed, and surgical duration also increases. Prodrome was noted in 4 obese and 4 overweight patients as per BMI in our series signifying that obesity is a risk factor especially if combined with any advanced indications for PTELD surgery.
The advantages of using a gravity flow system are the easy setup and maintenance as well as a low complication profile as the pressure rarely reaches dangerous levels inside the closed cavity, although it requires change in height of bottle as level of saline in bottle is decreasing [38]. This can be overcome by use of our IMP during surgery (Figure 2C). Irrigation fluid devices with an automated pump are commonly used to control pressure or flow volume. Use of automated pump for irrigation fluid can be associated with complication of fluid extravasation into potential spaces and adjacent soft tissue planes leading to edema in laryngeal, tracheal tissue which can cause airway obstruction and has been described in allied arthroscopic surgeries of shoulder [39].
In a study by Wu and Guan [40] comparing the effect of gravitational vs automated pump method in PTELD, a less surgical duration, intra-operative blood loss and low rate of complication in perfusion pump group has also been reported. They suggested use of automated pump with optimal pressure of 60–80 mmHg and fluid flow at 80–100 mL/min for clear intraoperative vision during PTELD. In one case each of our series, automated pressure pump and larger diameter working channel endoscope was used before a trial of procurement plan for institute. In the case with the automated pump the standard pressure recommendation was 150 mL/min [5]. Still, it led to prodrome, and it utilized 16.2 L of irrigation fluid. In a similar fashion in a case where larger diameter working channel (4.3 mm) the prodrome developed (11.4 L of irrigation fluid). The fluid used was more than usual in both. So, it is inferred that with FESS in LA, automated pumps are to be carefully used and larger channel endoscope will use more fluid to maintain better operating field.
Two studies from same institute reported about neck pain (n=8) in monitored quantified cervical epidural pressure in PTELD in irrigation with automated pump. Their average time of onset of neck pain was 17–52 minutes (34.37±11.71) [5,12]. This itself suggests the effect of an automated pump can be devastating. In our study the time of onset of prodrome was quite late in all the patients (n=8, 64.3 minutes) in gravity method with IMP. But, in our one patient with pump trial (3rd case), the symptoms started at 33 minutes (pump was then abandoned) and again after corrective measures and restarting re-developed prodrome at 79 minutes on carrying with gravity method with IMP protocol, before finally finishing successfully after the halt at 107 minutes from start of irrigation. In the same above quoted pioneering study with a basal irrigation speed of 150 mL/min, 17.39% (n=4/23) patients complained of neck pain, while with speed of more than 200 mL/min 80% (n=4/5) patients experienced neck pain [12]. In our single case of automated pump with 150 mL/min speed, we precipitated neck pain and that too quickly at 33 minutes. It was far below the 64.37 minutes average of our other 8 patients with prodrome.
Under normal circumstances, cerebral blood flow is usually tightly auto regulated. With rise in ICP, autoregulatory mechanisms of the brain will increase the blood pressure to maintain a normal cerebral perfusion pressure. This increase in the blood pressure will lead to more intra-operative bleeding, requiring surgeon to increase the fluid flow rate and increasing the duration of infusion causing an even higher ICP than before leading to vicious cycle [26]. Additionally, to reduce anxiety and pain under LA, the used conscious sedation medications can also result hypoventilation adding on raised ICP due to increasing intra-arterial carbon dioxide partial pressure [10,12].
Evolution of easier endoscopic spine surgery in form of unilateral biportal endoscopy which has separate viewing and working portal in surgical field has an advantage of allowing for outflow through a separate portal during continuous irrigation which can prevent stagnation and collection of fluid within epidural space and avoid significant rise in ETP [26].
In our study 3 patients who developed prodrome during surgery had concurrent episode of sudden increase in BP and tachycardia and one patient had bradycardia. These vital signs along with prodromal symptoms of discomfort, upper back pain, neck pain, stiffness and headache, which occurred in patients under LA during PTELD can facilitate early detection of seizures or potential ICP increases. Upper back pain/neck pain and discomfort-confusion was the commonest prodrome in our series.
When these red flag prodromes appears, all risk factors including anesthetic, surgical causes which can cause seizure should be checked and corrected immediately. Stopping the surgery, comforting the patient, managing vitals, giving anti-convulsant, restarting the surgery after 10–15 minutes, reducing infusion pressure and reducing further surgical time are the important key points in the prevention of further seizure. In all our patients, corrective measures worked and completion of surgery was possible. Two patients had repeat prodrome and needed a halt twice. Literature suggests starting surgery at low irrigation pressure with pump, quick surgery, and keeping epidural work towards the last of the surgery and avoiding inciting any bleed to prevent prodrome as preventive measures [9,12,26]. As the epidural space gradually fills, the shrunken dural sac loses its initial compensatory ability, and a steep increase in ETP is imminent likely. This is described as exhaustion of epidural compliance [12]. Even if temporary halting of surgery is done, the ETP may come to base line but can shoot again quickly. Therefore, experimentally it is suggested that more than 30 minutes are needed to regain its full compliance [12,27]. But every time in our current study we started the procedure in 12 to 15 minutes and accomplish the surgery and end points of decompression. Our correction measures were comforting the patient, managing vitals, giving anti-convulsant, restarting the surgery after a halt with reduced infusion pressure and reducing the further surgical time. In two of our cases, we still had to halt twice after 33/79 minutes and 26/59 minutes respectively (with further working time between the two prodromes as 46 minutes and 33 minutes in those two cases) and achieve the end points of decompression. So, practically corrective measures can work and give you time to complete the surgery. A completely contradicting report with tiring laborious experience of surgeon and patient with repetitions of wait-start-wait have been observed in attempts of quick resumption of surgery following prodrome [5,12]. But we believe that with our institute’s gravity method and IMP, we could still get away. This also justifies that non automated pump like ours doesn’t lead to exhaustion of epidural compliance.
The presence of fluid in epidural space was pathognomic finding in all the 9 cases. This fluid was present on 3.8 spinal segment levels proximally on average in our study. It signifies that a fluid presence circumferentially or more than 3 levels can be a risk factor. In regular operated cases this fluid level was never noted beyond 2 segment levels and post-operative MRI is a protocol for PTELD at authors institute for 14 years. The fluid egressing extra-canalicular, in irregular pattern was also noticed in 5 cases suggesting the extravasation of fluid in adjacent soft tissue due to excess ETP which was created.
All patients had adequate decompression, immediate functional outcome, and patient satisfaction index. At the follow up period of 9.65±9.32 months the evaluation of functional outcomes using VAS score leg/back, the patient satisfaction index and the Macnab outcome were good to excellent in all the patients in spite of the temporary episode.
The current study has many limitations. It was only a very small number of patients and retrospective in nature, but the number of cases is still the largest reported from a single center and looking to the rarity of seizures and methodological variations in execution at different institutes, large series of statistical significance will likely never get reported. This study is based entirely on speculation because none of the patients developed seizure, but classical prodrome features were present and thorough justification on imaging and clinical records could be done. We did not perform detail MRI studies post-operative, but only T2 sagittal and axial studies. The acquired MR images were within 3 hours of surgery and substantial time delay would have given the fluid a chance to egress into normal circulation. Per-operative MRI like X-MRI is suggested for more better imaging. But it was noted that in one of the studies of Choi et al. [5] reporting the seizures, the MRI images showed Y shaped severely exsanguinated dural sac. These were visibly extreme compressions. Also, the images were acquired in the supine position as against a surgical prone position. It’s possible that the fluid position shifts in the supine decubitus position. The pathological conditions of surgical indications in our study are too less in number to have statistical significance. More of anecdotal evidence can be argued. The technical postulates on 4.3 mm working channel endoscope and automated pressure pump were made on just one case each and there may be unintended technical fallacies while execution by author due to non-familiarity. The irrigation method was non-standardized though commonly used gravity method. Moreover, the IMP was again, an error prone method to document and cannot be objectified to true pressure values to arrive at linear correlations. No electro encephalogram and MR scan of brain was done immediate to confirm impending electrical activity or identify pathological changes in MRI brain. Finally, the results of the current study may not be generalizable to patients operated by PIELD or UBE. But, still looking to the rarity of the seizure complication and pure intentions to avoid it, the accepted prodromes, risk factors and technical knowhow discussed here will contribute to the literature in the exponentially evolving field of FESS. Available literature is tabulated with salient description and remarks (Table 4).
CONCLUSION
Occurrence of prodromal symptoms should be considered as red flag sign for avoidable seizure activity and alert surgeon during PTELD. Duration of surgery and infusion fluid flow rate are associated controllable risk factor during surgery. Potential factors increasing them should be born in mind. Anecdotal reports don’t constitute evidence, further large data pooling is needed for conclusive guidelines.
Notes
Ethical statements
All the procedure involving human participant were in accordance of 1964 Helsinki declaration and its later amendments. This is an retrospective observational study and therefore, does not need an ethical committee approval.
Conflicts of interest
Ajay Krishnan is the Editor of the Journal of Minimally Invasive Spine Surgery and Technique and was not involved in the review process of this article. All authors have no other potential conflicts of interest to declare relevant to this article.