Complications of Minimally Invasive Transforaminal Lumbar Interbody Fusion
Article information
Abstract
Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) is a popular surgical technique for treating lumbar spinal disorders. While it offers advantages over traditional open surgery, it is not without complications. The prevention, early detection, and proper management of complications are crucial in achieving successful outcomes with MI-TLIF. Patient selection, surgical technique, and postoperative monitoring play key roles. Advances in imaging, tools, and implant design contribute to reducing complications. Surgeons should be aware of the potential complications associated with MI-TLIF and take appropriate measures. Understanding and addressing these complications will lead to better patient outcomes and long-term success. Meticulous surgical technique, proper patient selection, preoperative planning, and intraoperative monitoring can help mitigate these complications.
INTRODUCTION
Lumbar spinal fusion treats diverse spinal pathologies that cause spinal instability. Realizing the need for a less invasive lumbar spinal fusion procedure with fewer complications, Foley and Lefkowitz adopted the principles of minimally invasive spine surgery (MISS) and introduced the minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) in the early 2000s [1-5].
The MI-TLIF has shown reduced complications, decreased intraoperative blood loss, shorter hospital stays and recovery time, and decreased postoperative narcotic usage while maintaining similar clinical outcomes and fusion rates to conventional open TLIF since its inception [6-19]. The success of MI-TLIF can be attributed to the following fundamental principles: (1) minimizing damage to soft tissues and avoiding destabilization of the spinal segment(s) to achieve the surgical goal with the smallest possible operative footprint; (2) utilizing a unilateral approach to achieve bilateral decompression when needed; and (3) achieving neural decompression indirectly.
Although there is no definitive definition of MI-TLIF, the lack of a precise definition has led to significant variations in how surgeons perform the procedure due to various technical nuances associated with each step. To tackle this issue, Lener et al. [20] conducted a systematic review in 2020 and identified several commonly agreed-upon patterns for performing MI-TLIF among most MIS surgeons. These include the utilization of paramedian incisions, a tubular retractor for total facetectomy, decompression, interbody cage placement, percutaneous insertion of pedicle screw rod constructs with intraoperative imaging, and the use of a microscope or endoscope is required for adequate visualization and illumination given the narrow work corridor for this procedure. On the other hand, approaches that involve expandable nontubular retractors requiring extensive subperiosteal dissection from the midline laterally or the use of wide pedicle-to-pedicle exposure with specular-based retractors are less likely to be considered as part of the minimally invasive surgery (MIS) approach. When performing MI-TLIF, it is crucial for surgeons to exercise caution and be aware of various contraindications. These contraindications, which are not exhaustive, encompass conditions such as extensive epidural scarring, arachnoiditis, active infection, conjoined nerve roots (which can hinder access to the disc space), and osteoporosis in patients. Surgeons should be mindful of these factors to ensure appropriate patient selection and minimize potential risks during the procedure [21].
The term "complication" carries different technical and emotional significance for various stakeholders, including patients, surgeons, medical boards, the legal community, and others involved. Sokol and Wilson [22] proposed a four-part definition of “surgical complication”: A surgical complication is any undesirable, unintended, and direct result of an operation affecting the patient that would not have occurred had the operation gone as well as reasonably hoped.
While the complications of open TLIF are well-known, there is still a lack of understanding regarding the complications specifically associated with MIS techniques. The present review aims to identify and classify potential complications associated with MI-TLIF.
CLASSIFICATION
There is a paucity of literature when classifying, describing, and reporting complications of MI-TLIF. To address this, Patel et al. [23] proposed a descriptive classification system for perioperative complications of the MI-TLIF procedure, categorizing them into early complications (occurring within 6-month postsurgery) and late complications (occurring after 6-month postsurgery) within 5 broad categories. Building upon this classification, we have further expanded it and included additional subcategories, as in Table 1.
EARLY PERIOPERATIVE COMPLICATIONS
1. Infection
All surgical procedures carry the inherent risk of infection, which can have significant consequences for the patient. Surgeons primarily focus on wound infections and their potential complications, but infections affecting the urinary tract, respiratory system, and gastrointestinal tract are also causing concern. Introducing foreign materials into the patient's body has increased the risk of immediate postoperative infections and the possibility of late infections caused by hematogenous implant seeding. The introduction of MI-TLIF has led to a notable decrease in surgical site infection (SSI) incidence. This can be attributed to tubular retractors inserted through gradual soft tissue dilation without requiring extensive subperiosteal muscle dissection [24]. This approach minimizes soft tissue trauma and reduces the dead space within the surgical site, thereby minimizing the exposed surface area during the procedure. These factors contribute to the reduction in SSI rates associated with MI-TLIF.
Multiple systematic reviews and meta-analyses provide further evidence to support this observation [25-28].
O'Toole et al. [24] conducted a study and discovered that surgical wound infections were significantly lower in MI-TLIF (0.6%) than in open TLIF procedures (4.0%). The reduction in the incidence of wound infections observed in the study resulted in a direct cost savings of $98,974 per 100 MI-TLIF procedures performed. Shousha et al. [29] analyzed 4,350 MIS procedures and concluded that the infection rate after posterior transtubular microscopic assisted spinal surgery is very low (0.09%). Surgical debridement with fusion was the method of choice in treating such complications. This minimally invasive technique reduces markedly the risk of postoperative infection compared with other large series published in the literature. Wong et al. [30] reported a low incidence of surgical wound infection (0.2%), which compares favorably to the experiences reported by other authors. To further reduce infection rates in MI-TLIF, they recommended avoiding placing fingers directly into the surgical wound, as microscopic breaks in surgeons' gloves could potentially increase the risk of surgical wound infections. Additionally, the study observed 7 perioperative medical infections (1.3%), including urinary tract infections and pneumonia. Notably, revision and multilevel procedures had a higher rate of perioperative infections than first-time MI-TLIF. Wang et al. [31] conducted a study aiming to develop and validate supervised machine learning algorithms to predict the risk of SSI following MI-TLIF. Through stepwise logistic regression analyses, the study identified several potential predictors of SSI, including preoperative glycated hemoglobin A1c levels, estimated blood loss (EBL), preoperative albumin levels, body mass index (BMI), and age. These variables were associated with the risk of developing SSI in MI-TLIF.
2. Cardiopulmonary Complications
Cardiopulmonary complications, such as deep vein thrombosis (DVT) and pulmonary embolism (PE), are uncommon after MI-TLIF, with reported incidence rates ranging from 0.4% to 4.0% [11,30,32-34]. In a systematic review and meta-analysis by Bernatz and Anderson [35], DVT/PE was identified as the cause of 30-day readmissions in only 2 out of 13 studies. Although infrequent, DVT/PE can have devastating consequences, as evident from a study by Wong et al. [30], where one patient out of 513 undergoing MI-TLIF died from a massive PE, while 6 others required anticoagulation therapy for DVT or PE.
While the incidence of DVT/PE after MI-TLIF is not higher than that of standard TLIF, some researchers speculate that the longer operative time associated with MI-TLIF may be a contributing factor [11]. However, further research is needed to fully comprehend the relationship between operative time and the risk of DVT/PE in MI-TLIF procedures. Olinger and Gardocki supported the concept of a learning curve in MI-TLIF, noting a decrease in operative times with experience exceeding 100 cases, thus decreasing the incidence of DVT/PE. They also emphasized using calf sequential compression devices (SCDs) during the procedure, as this preventive measure played a role in the absence of DVT/PE complications. Their findings suggest that as surgeons gain more experience and implement preventive measures like calf SCDs, the risk of DVT/PE can be minimized during MI-TLIF procedures. Adhering to the guidelines established by the North American Spine Society, all patients in the study received immediate postoperative treatment to prevent thromboembolism. This included using a thromboembolism-deterrent hose and mechanical prophylaxis using calf SCDs. Notably, no pharmacologic DVT prophylaxis was administered as part of the postoperative management protocol.
3. Neurological Injury
Whether MI-TLIF is associated with a higher incidence of nerve root injury has been widely discussed. The MI-TLIF approach allows for an aggressive discectomy and increases lateral angles for interbody cage placement while minimizing the need for thecal sac or nerve root retraction. However, concerns arise due to the smaller corridor used in MI-TLIF compared to the conventional open approach.
Conventionally, nerve injuries can occur due to misplaced screws or during cage insertion. In the literature, the reported incidence of postoperative neurological deficits in patients undergoing open TLIF is approximately 1%–3% [36]. In comparison, the reported incidence of neurological deficits after MI-TLIF ranges from 0.7% to 9.5% [23,30,37-39]. It is important to note that these neurological deficits are often transient and frequently associated with malpositioned hardware.
Hu et al. [32] in their systematic review comparing complications in MI-TLIF and Open TLIF, found no significant difference in the incidence of neurological injuries between the 2 approaches. It is important to consider the advancements brought about by magnification and illumination techniques in MI-TLIF, which have revolutionized the procedure and contributed to decreased complications.
To reduce the incidence of neurological injuries, several principles should be followed. First, ensuring adequate visualization of the exiting and traversing nerve roots during the procedure is crucial. This facilitates meticulous dissection and mobilization of the thecal sac, as well as the exiting and traversing nerve roots. Second, the use of navigation or robotic guidance for percutaneous insertion of pedicle screws can enhance precision and reduce the risk of nerve injury. Third, inserting expandable interbody cages, which have a smaller footprint at the mouth of the disc space and expand to the desired size within the disc space, can help minimize the risk of nerve compression by avoiding "overstuffing" the disc space with an oversized interbody cage, as this can directly injure the nerve roots or cause postoperative radiculopathy due to indirect stretching of the nerves. Finally, all fusion procedures should consider intraoperative neurophysiological monitoring to provide real-time feedback and early detection of potential nerve-related complications. Stimulated electromyography is an intraoperative neurophysiological monitoring technique to help determine iatrogenic nerve injury resulting from malpositioned screws. Any stimulus less than 8 mA raises concerns for possible malposition. Furthermore, a stimulus of 4–5 mA has a specificity of close to 100% for a pedicle wall defect [40].
To address the concerns of durotomy and nerve root injury during MI-TLIF, Wang et al. [41] proposed a modified technique where the surgical order was altered. In this modified approach, the interbody fusion procedure is performed before the decompression procedure. By performing the interbody fusion first, the surgeon can create a stable foundation and restore disc height while being protected by the ligamentum flavum (LF). This step is followed by the decompression procedure, which involves the removal of any compressive structures, such as LF, herniated discs or bone spurs. The findings suggest this modified approach is safe and effective, potentially reducing durotomy and nerve root injury during MI-TLIF.
By adhering to these principles, surgeons aim to mitigate the risk of neurological injury during MI-TLIF and enhance patient safety. Continued advancements in surgical techniques and technology are likely to improve outcomes further and reduce the incidence of complications in MI-TLIF procedures.
4. Durotomy
Incidental durotomy during degenerative lumbar spinal surgery is a common complication. However, its reported incidence in the literature varies widely, ranging from 0.3% to 30%, depending on the specific type of surgery [42-46]. An incidental durotomy can lead to various complications, such as postural headaches, persistent cerebrospinal fluid (CSF) leakage, and the formation of pseudo meningoceles [47-50]. These complications can significantly affect patient recovery and may necessitate further interventions.
Supporters of MI-TLIF highlight its advantages, including a muscle-sparing approach, smaller incisions, and reduced dead space. These factors are believed to prevent pseudo meningocele formation and persistent CSF leaks following durotomy by acting as a natural barrier [50].
Several risk factors have been identified for dural tears, including revision surgery, diabetes mellitus, high BMI, female sex, and advanced age [51-53]. These factors should be considered during surgical planning and patient counseling to minimize the risk of durotomy. Epidural fibrosis is an important consideration during revision surgeries. Many authors have described the minimally invasive extraforaminal lumbar interbody fusion (ELIF) technique to address this concern [54-57]. ELIF involves creating a working corridor angled at approximately 45° relative to the midline. This angle allows for avoiding the dural sac and postoperative epidural fibrosis in a lateral direction, thereby reducing the risk of dural tears. The procedure is performed using an expandable tubular retractor system, which facilitates atraumatic access by following the natural intermuscular cleavage plane between the multifidus muscle and the longissimus thoracis muscle pars lumborum. One notable advantage of the ELIF technique is that the muscles involved do not exhibit signs of atrophy or fatty degeneration postoperatively. This suggests that the approach has a relatively minimal impact on the surrounding musculature. ELIF is ideal when there is foraminal compression in revision cases. In cases where intracanal lesions are the target of the procedure, partial removal of the superior facet is often sufficient to achieve the desired access and decompression. Overall, the ELIF technique offers a surgical approach to minimize the risk of complications associated with epidural fibrosis and durotomy during revision surgeries. By utilizing a specific working corridor and atraumatic access, this technique provides a potential solution to mitigate these concerns.
A technique assessment published by Boukebir et al. [58] observed that most durotomies occur during the caudal and contralateral dissection while removing thickened LF from the underlying dura. To minimize the risk of durotomy, certain techniques can be employed. One important technique is carefully separating the dura from the LF using a ball-tip instrument before removing the LF with Kerrison rongeurs. This step ensures that the dura is adequately protected during the removal process. Additionally, 90° Kerrison rongeurs can help minimize the risk of durotomy due to their specific design and angle. Another useful technique involves closing the Kerrison rongeurs and moving them slightly to the right and left before committing to a bite. This maneuver allows for the assessment of any significant movement of the dura. If the dura has inadvertently been included in the bite, it will move noticeably during this maneuver. More significant dural tears can be avoided by being attentive to this movement. These techniques aim to enhance the precision and safety of the surgical procedure, reducing the occurrence of durotomy during the removal of the LF.
In cases where a dural tear occurs during the procedure, the management approach depends on the size of the defect and whether or not nerve roots are protruding through the tear. Here are the general steps taken to address dural tears:
(1) Small defects with contained nerve roots: If the dural tear is relatively small and the nerve roots remain contained within the thecal sac, the defect is covered with fibrin glue or DuraSeal after achieving hemostasis. This step is performed before the removal of the tubular retractor.
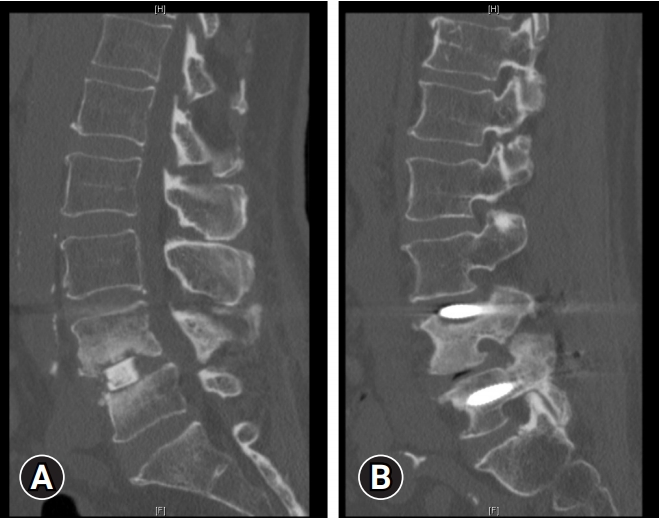
(2) Large defects with extruded nerve roots: In cases where the accidental durotomy is larger and nerve roots are extruding through the defect, a primary repair is performed. The goal is to place the nerve roots back into the thecal sac. Adequate drainage of CSF is important in facilitating this process. Figure 1 shows an example.
An example of a large accidental durotomy with nerve roots extruding through the defect. Primary repair was performed, with the goal of placing the nerve roots back into the thecal sac.
(3) Repair technique: The surgical team may utilize the Scanlan endoscopic dural repair set and a 4-0 Nurolon TF-5 "fishhook" suture suitable for dural repair. This suture has been successfully used through small tubes as small as 15 mm in diameter. A Valsalva maneuver is performed to confirm the watertight closure of the repair.
(4) Additional coverage: Once the repair is completed, the site is covered with fibrin glue or DuraSeal for further reinforcement.
After the repair, patients with CSF leaks are typically placed on flat bed rest until the following morning and then mobilized early, within the first 24 hours following surgery [59]. This approach helps promote healing and prevents complications associated with CSF leaks.
It is important to note that the specific management of dural tears may vary depending on the surgeon's preference and the individual patient's condition.
5. Instrumentation Complications
Pedicle screw malposition and interbody cage migration are recognized as common early perioperative instrumentation complications in MI-TLIF. The reported incidence of screw malposition varies in the literature, and corrective measures are typically taken intraoperatively to address any observed malposition.
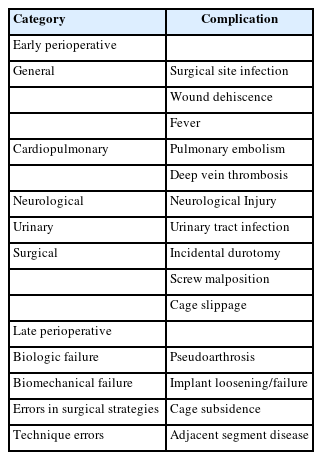
Patel et al. [23] and Hu et al. [32] reported incidences of screw malposition ranging from 0.35% to 1.2%. Figure 2 shows an example of screw malposition violating the medial pedicle wall. Additionally, Wong et al. [30] reported a 1.2% incidence of intraoperative fracture of the guidewire. Figure 3 is an example of a k-wire fracture. However, it's important to note that the findings and reported incidences may be influenced by factors such as the heterogeneity of the study populations, variations in surgical techniques, and the specific criteria used to define and assess screw malposition.
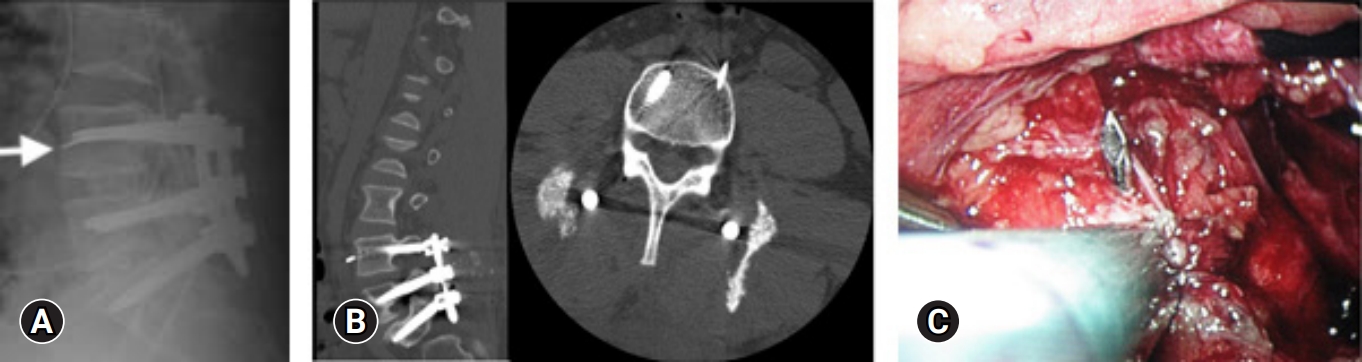
A 43-year-old man underwent minimally invasive transforaminal lumbar interbody fusion, but his left L4 k-wire broke in 2 places. (A) It was retained (arrow). (B) Postoperative computed tomography showed that the fragment was adjacent to the left internal iliac artery. (C) A laparoscopic transperitoneal removal of the k-wire fragment was performed on postoperative day 6.
El-Desouky et al. [60] conducted a study to evaluate the placement of percutaneously inserted pedicle screws in MI-TLIF using intraoperative 2-dimensional fluoroscopy and 1-year follow-up computed tomography (CT) scans. They reported a pedicle wall violation rate of 13.97%, with only 4.31% of the screws showing violations exceeding 2 mm. Of all the screws, only 0.48% led to complaints and subsequent reoperation involving 5 patients. The study observed increased pedicle violation rates in the upper lumbar pedicles. And for S1 screws, violations were 2.68 times more common on the left pedicle screws. To address this startlingly high incidence, the screw position should be carefully assessed, ensuring that the screw does not cross the midline on the anteroposterior view and respects the superior and inferior limits of the pedicles on the lateral view. Additional strategies include palpating the pedicle walls with a hook and directly visualizing decompressed roots to enhance accuracy during screw placement.
The findings underscore the significance of precise screw placement and the utilization of intraoperative imaging techniques to minimize pedicle violations and improve patient outcomes. The introduction of advanced technologies such as intraoperative 3-dimensional (3D) CT, computer-assisted navigation, and robotics has substantially decreased the incidence of these complications. Intraoperative navigation systems offer several benefits, including reduced radiation exposure for the surgeon. By providing real-time guidance, these systems enhance the accuracy of screw placement. Moreover, intraoperative CT scans or 3D fluoroscopy allows for a comprehensive evaluation of the screws, enabling the detection of any potential issues and the possibility of making corrections before the procedure concludes.
The findings from a systematic review and meta-analysis by Shin et al. [61] provide evidence that computer-navigated screw insertion has a lower risk of pedicle screw perforation than conventional freehand insertion. The overall risk of perforation was 6% with navigation and 15% with conventional insertion. Notably, no neurological complications were reported with navigated insertion, while 3 were observed in the nonnavigated group. These results suggest that navigation can improve the safety of screw placement. In a study conducted by Dea [62], a patient-level data cost-effectiveness analysis was performed, leading to the conclusion that computer-assisted spinal surgery can lower reoperation rates, thereby carrying significant cost-effectiveness implications with potential policy implications as well. While the acquisition and maintenance costs of this technology are relatively high, the study suggests that these expenses can be balanced by the considerable costs associated with reoperations using conventional fluoroscopy methods. The cost-effectiveness analysis indicated that, particularly for high-volume centers with comparable case complexity to the population studied, the adoption of computer-assisted spinal surgery is economically justified. In addition, the systematic review of Naik et al. [63] indicated that robot-assisted placement had fewer complications than freehand placement. The odds of complications were higher with freehand placement compared to robot-assisted and CT-navigation placement.
Both studies further support using navigation and robotic assistance in spinal surgery to minimize complications and enhance patient safety. Furthermore, the meta-analysis did not find a significant difference in total operative time and EBL between navigated and nonnavigated procedures, suggesting that navigation does not significantly impact these aspects of the surgical procedure.
The increased accuracy in screw placement achieved through these technologies may also allow for larger diameter pedicle screws, which can enhance the mechanical strength of the spinal construct. Further investigation is warranted to study these advancements' mechanical properties and long-term outcomes.
Improvements in navigation software and the availability of intraoperative CT scanners hold promise for enhancing navigation capabilities, making them more user-friendly and accurate. Integrating advanced imaging technologies and real-time feedback can further improve the precision and safety of surgical procedures.
The risk of interbody cage migration after MI-TLIF is a concerning complication in a small percentage of cases, occurring in a range of 0.5% to 3.8% within the first 6 months postoperatively [23,30,64,65]. Hu et al. [65] identified posteriorly located and undersized cages as major risk factors for posterior cage migration based on their analysis of 953 cases. To minimize the risk of cage migration, several steps can be taken. Maximizing the dimensions of the interbody cage during placement, particularly while distracting the contralateral side, can help prevent potential migration. The interbody cage trial should fit snugly and require significant effort to remove, ensuring a secure placement. Using a flat or curved tamp to turn the interbody cage within the disc space can alter its trajectory and potentially reduce the likelihood of migration. Expandable cages can also address this issue, as they can be inserted in a compact form through the tubular retractor and then expanded within the disc space, providing a secure fit. Lastly, applying bilateral compression across the surgical levels can promote fusion and reduce the potential for cage migration.
LATE PERIOPERATIVE COMPLICATIONS
Etiopathology of Late perioperative complications in MI-TLIF can be multifactorial and arise from failures in biology, biomechanics, surgical strategy, or technique errors. Most complications result from a combination of these factors; in some cases, failure in one area can increase the risk of a complication in another.
For example, if there is inadequate fusion or poor bone quality, it can lead to implant failure or pseudarthrosis. Biomechanical factors such as improper load distribution or excessive stress on the instrumentation can contribute to hardware failure or adjacent segment degeneration. Surgical strategy errors, such as inadequate decompression or inappropriate cage selection, can result in persistent neural compression or cage-related complications. Technique errors during screw placement, interbody cage insertion, or soft tissue handling can lead to pedicle violation, nerve root injury, or vascular complications. Insufficient training or experience in MIS techniques can also increase the risk of complications.
To minimize these complications, it is crucial to have a comprehensive understanding of the underlying pathology, employ appropriate surgical strategies, use meticulous surgical techniques, and ensure proper patient selection.
1. Fusion-Related Complications
A successful MI-TLIF heavily relies on establishing a solid interbody fusion, given the challenges associated with achieving a posterolateral fusion due to limited exposure. To prevent pseudarthrosis, it is crucial to thoroughly comprehend the biological and biomechanical considerations of fusion surgery. There are 3 fundamental requirements necessary for achieving bony fusion:
(1) A sufficient amount of osteogenic cells, an osteoconductive matrix that can act as a scaffold for new bone to form and osteoinductive signals that promote bone formation.
(2) It is also imperative to have adequate blood supply to the fusion bed.
(3) Proper biomechanical stability also aids in limiting micromotion and strain to allow for bony fusion.
Variations in the literature regarding MI-TLIF techniques primarily revolve around the selection of implants and graft materials used for interbody fusion. These implants play a crucial role in providing biomechanical stability and maintaining load compression on the endplate-graft complex, thereby facilitating successful arthrodesis. Different options for grafts and fusion enhancers encompass a range of materials, including autologous bone obtained from laminectomy and facetectomy sites, autologous bone harvested from the iliac crest, allograft bone from cadaver donors, nonhuman bone substitutes such as hydroxyapatite and ceramics, demineralized bone matrix (DBM), and recombinant human bone morphogenetic proteins (rhBMPs).
The pseudarthrosis diagnosis is based on a combination of clinical symptoms and radiographic evidence obtained at the 1-year follow-up. Clinical symptoms suggestive of pseudarthrosis included persistent radiculopathy or axial back pain. Radiographic assessment using CT is performed for patients exhibiting these clinical symptoms and demonstrating radiographic indicators suspicious of pseudarthrosis like increased motion across the fusion segment, radiolucency around the instrumentation, settling of the interbody cage, or the absence of sentinel signs on lateral radiographs. Figure 4 shows an example of pseudoarthrosis with screw loosening.
Parajón et al. [66] conducted a comprehensive meta-analysis to compare the fusion rates associated with different graft materials used in MI-TLIF. Their study included a diverse range of cases with varying graft material combinations. The findings revealed notable trends in the utilization and fusion rates of specific graft materials.
Among the cases analyzed, rhBMP was utilized in 36.5% of all MI-TLIF cases. Furthermore, the combination of local autograft with rhBMP was used in 21.9% of cases, while the combination of local autograft with bone extenders and rhBMP was employed in 14.6% of cases. Notably, there were no instances of iliac crest bone graft combined with rhBMP.
Regardless of the graft material used, the fusion rates for MI-TLIF were consistently high, exceeding 90%. The combination of autologous local bone with both bone extenders and rhBMP demonstrated the highest fusion rate, reaching 98.8% at the 12-month follow-up. On the other hand, the isolated use of local bone yielded the lowest fusion rate of 91.8% at the 12-month follow-up. This discrepancy in fusion rates may be attributed, in part, to the relatively lower volume of local bone obtained from MI-TLIF procedures compared to open techniques.
The safety profile of rhBMP has been a subject of scrutiny and has been investigated in numerous previous studies. While rhBMP2 has demonstrated the ability to increase fusion rates in spinal fusion procedures, concerns have been raised regarding its potential adverse effects, such as hyperostosis and inflammation [67]. Notably, there have been reports suggesting a potential association between rhBMP and de novo cancer formation, particularly when administered at doses exceeding 40 mg[67]. However, the exact risk of postoperative cancer development following the use of BMP in spinal fusion has not been definitively established [68], and the evaluation of this potential side effect should be conducted on an individual basis for each patient by the surgeon.
Conversely, studies have shown that utilizing rhBMP at lower doses, specifically less than 5 mg per level, has yielded favorable fusion rates and positive clinical outcomes during long-term follow-up. It is worth noting that the concentration of rhBMP available can vary from 1 to 12 mg, and the decision regarding its application is determined by the treating surgeon. This variation in concentration and the individualized approach to dosage based on the surgeon's judgment makes it challenging to quantify the precise dosage per surface area.
Other complications documented by rhBMP use are symptomatic ectopic bone formation, vertebral osteolysis, recalcitrant postoperative radiculopathy, and pseudarthrosis. Potential causes include improper dosage and a closed space that prevents the egress of the postoperative BMP-2 fluid collection [69-71].
2. Adjacent Segment Disease
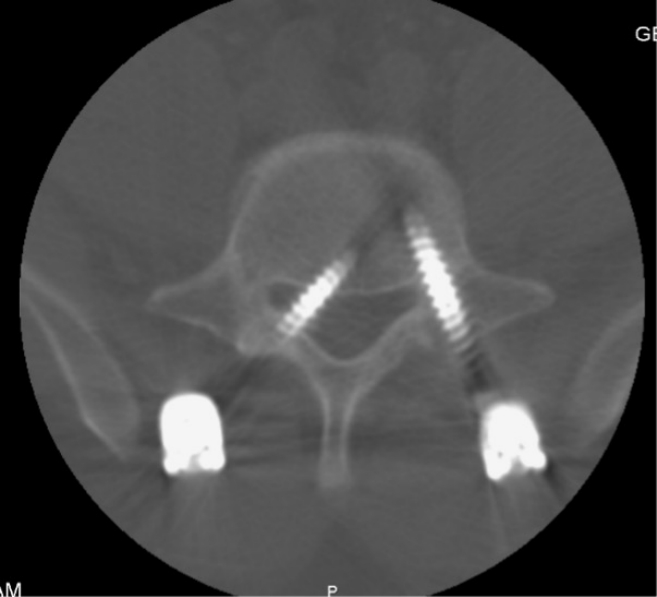
Adjacent segment degeneration refers to radiographic changes observed in the intervertebral disc adjacent to a previously operated spinal level, irrespective of symptoms. When symptomatic, it is referred to as adjacent segment disease (ASD)[72]. Figure 5 shows a 15-month postoperative lateral radiograph showing L5–S1 cage subsidence, and L4–5 adjacent segment disease (arrow). The reported incidence of reoperation rates for adjacent segment degeneration after lumbar fusion surgery ranges from 3.5% to 14.5% [73-75].
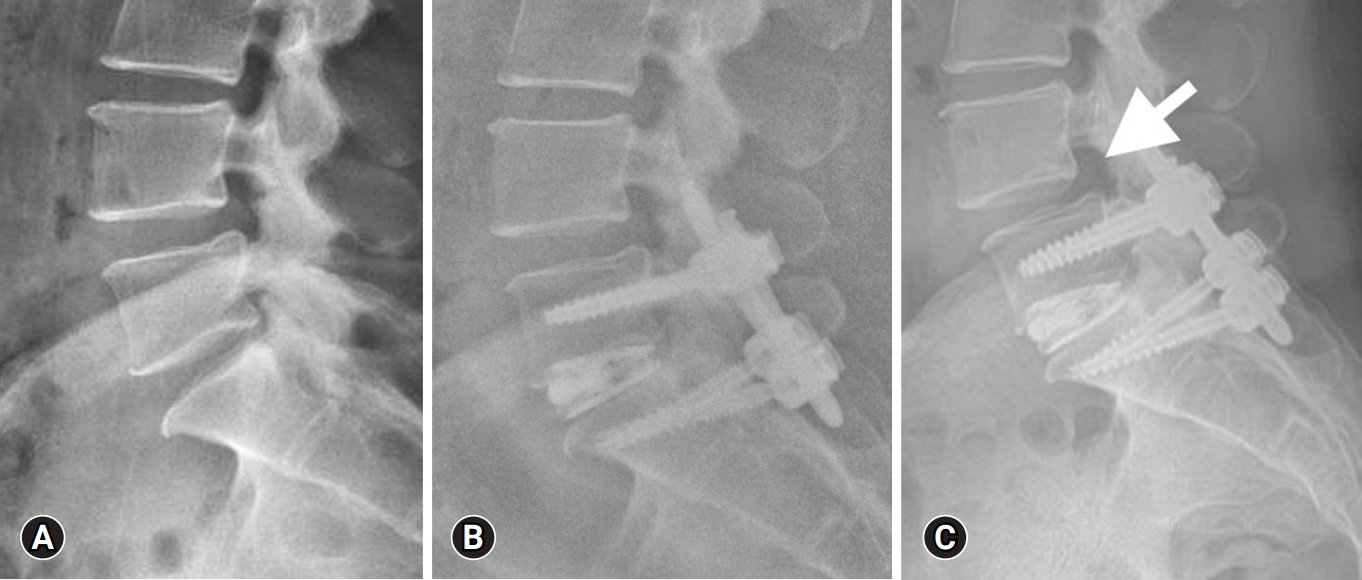
An example of cage subsidence at 15 months postoperative with adjacent segment spondylolisthesis. Preoperative lateral radiograph (A), 1-month postoperative lateral radiograph (B), and 15-month postoperative lateral radiograph (C) showing L5–S1 cage subsidence, and L4–5 adjacent segment disease (arrow).
Yuan et al. [73] conducted a multivariate analysis and identified several independent risk factors for developing ASD after MI-TLIF, including lower bone mineral density (BMD), higher BMI, and preoperative adjacent segment disc degeneration. While Hashimoto et al. [76] included age over 60 years, high BMI, long fusion segment, excessive distraction at the fusion segment, hypo lordotic fixation, retroverted pelvis, Pfirrmann's disc degeneration grading of more than 3 in the adjacent segment, osteoporosis, and facet/facet capsule damage.
Ever since Lee et al. [77] initially reported a correlation between facet violation and ASD, there is increasing attention on the role of superior facet joint violation. However, conflicting evidence exists regarding whether MI-TLIF procedures increase the incidence of facet violation.
Lau et al. [78] conducted a study suggesting that minimally invasive pedicle screw placement is not associated with higher rates of facet violation. However, this finding was contradicted by Patel et al. [79], who demonstrated that iatrogenic superior facet violation is more prevalent in MI-TLIF compared to open TLIF procedures at a single lower lumbar level.
Given these conflicting findings, it is advisable to adopt a cautious approach and consider the risk factors associated with facet violation, as suggested by Zhao et al. [80]. Specifically, a BMI of 30 kg/m2 or higher and pedicle screw placement at the L5 level were identified as independent risk factors for superior facet violation. Additionally, facet violation was more likely to occur in hypertrophic facets (with axial, sagittal, and coronal diameters ≥12 mm) or in cases where the facet joint had a coronal orientation (facet angle ≥40°).
In a meta-analysis involving 6 trials and 408 patients who underwent single-level lumbar interbody fusion, Li et al. [81] concluded that the incidence rate of ASD was lower in the MIS group than in those who underwent open surgery.
These findings suggest that MI-TLIF may be associated with lower rates of ASD and degeneration than open surgery. Identifying and considering various risk factors can aid in assessing the likelihood of developing ASD and help guide surgical decision-making and patient management.
3. Late Instrumentation Complications
Late instrumentation complications encountered in lumbar fusion surgeries include cage subsidence, screw loosening, and implant fracture. These complications can occur independently or, more commonly, form a spectrum of events that occur in conjunction with one another. The subsidence of the interbody fusion cage can gradually reduce intervertebral disc height. This reduction negatively impacts the anterior support of the spine, impeding successful fusion. Over time, this can lead to screw loosening as the stability of the construct is compromised. Ultimately, the combination of cage subsidence and screw loosening may progress to implant fracture, further compromising the integrity of the instrumentation used in the fusion surgery.
Cage subsidence in lumbar fusion surgeries is typically defined as the sinking of a cage into an adjacent vertebral body by more than 2 mm [82]. Figure 5C shows an example of 15-month postoperative L5–S1 cage subsidence. Several factors have been identified as being associated with cage subsidence. These include higher BMI, increasing severity of multifidus muscle atrophy, the specific type of cage (such as titanium-coated polyetheretherketone [PEEK] vs. PEEK), larger cage size (height ≥ 12 mm), the use of bone substitute (such as DBM mixed autograft versus pure autograft), and the posterior position of the cage [83].
The reported incidence of cage subsidence varies widely in the literature, ranging from 15% to 70% [33,83-86].However, it is important to note that the literature lacks clarity regarding the percentage of patients who experience clinical symptoms specifically attributed to cage subsidence.
Expandable cages in MI-TLIF have become increasingly common due to their benefits, such as a smaller insertion footprint and compact fit. However, using expandable cages introduces a distraction force in the disc space, which may not be an ideal environment for fusion. Consequently, multiple studies have raised concerns about the efficacy of expandable cages. While comparing the clinical and radiologic outcomes of patients undergoing MI-TLIF with expandable cages versus nonexpandable cages, these studies have shown that there may not be a significant difference in various parameters between the 2 types of cages [87-90]. These findings suggest that expandable cages may not provide substantial advantages over nonexpandable cages regarding patient outcomes. Moreover, the higher cost associated with expandable cages raises questions about their cost-effectiveness. Thus, it becomes unclear whether the added expense of expandable cages is justified.
Further research and long-term studies are needed to better evaluate the clinical efficacy, cost-effectiveness, and potential complications associated with expandable cages in MI-TLIF.
As indicated in previous studies, osteoporosis has been identified as a significant risk factor for cage subsidence. The stability and failure load of the endplate and cage are influenced by BMD. Earlier research has demonstrated a relationship between BMD and the likelihood of subsidence [91].
Oh et al. [92] conducted a study and observed a weak correlation between BMD and subsidence. They discovered that patients with a BMD score of less than -3.0 exhibited an increased risk of subsidence. Similarly, Cho et al. [93] conducted a study and reported that patients with a T score of -2.5 or lower had a sedimentation rate increase of at least -1.0 compared to patients with a higher T score.
These findings highlight the impact of BMD on the occurrence of cage subsidence. Patients with lower BMD scores, particularly those with a T score of -2.5 or lower, appear to be at an elevated risk of subsidence. This emphasizes the importance of osteoporosis and BMD assessment in the preoperative evaluation of patients undergoing lumbar fusion surgeries. Identifying patients with low BMD allows for appropriate measures to be taken to enhance stability and prevent complications associated with cage subsidence.
CONCLUSION
While MISS has demonstrated lower complication rates than traditional open methods, it is important to acknowledge that it still carries a unique set of complications. The complications can vary depending on the minimally invasive procedure performed and the underlying indication. The current published literature has identified several complications associated with MI-TLIF.
Adherence to meticulous surgical technique, proper patient selection, preoperative planning, and intraoperative monitoring can help mitigate these complications. Continued research and experience in the field of MISS are essential for further understanding and optimizing patient outcomes while minimizing the occurrence of complications.
Notes
Conflicts of Interest
Dr. Roger Härtl is a consultant for DePuy Synthes Spine and Brainlab. The other authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.