Strategies to Improve Neurological Safety in Full-Endoscopic Lumbar Fusion Surgery: A Comprehensive Review
Article information
Abstract
Full-endoscopic spinal fusion surgery has emerged as a crucial approach for managing lumbar degenerative spinal disease. A significant concern in endoscopic spinal fusion relates to the vulnerability of neural structures, particularly the risk of nerve root injury (NRI). This comprehensive review evaluates the critical importance of preserving neurological integrity during endoscopic spinal fusion procedures, focusing on multifactorial contributors to risk and effective strategies for safeguarding the nerve root. The review thoroughly examines anatomical considerations, surgical techniques, the utilization of specialized intraoperative instrumentation, and intraoperative monitoring as key factors influencing the risk of NRI. Understanding these variables is paramount for minimizing postoperative neurological complications and improving patient outcomes. The article succinctly summarizes the clinical presentation of nerve root injuries and recommends therapeutic interventions. It also discusses strategies for preventing NRI, emphasizing both preoperative considerations and intraoperative measures. This comprehensive review provides spine surgeons with valuable insights, highlighting the significance of meticulous techniques and preventive measures to optimize patient safety and overall surgical success in the context of endoscopic spinal fusion.
INTRODUCTION
Low back pain and radiculopathy, attributed to lumbar degenerative disease, has a historical background spanning over 2 centuries [1]. Surgical intervention, such as posterior decompression with or without lumbar interbody fusion (LIF), becomes an option when conservative treatments offer minimal relief [2,3]. Reflecting on the historical progression, the concept of formal LIF surgery via the posterior route was initially reported in 1944 by Briggs and Milligan [4] and, the notion of transforaminal access emerged with the first publication in 1982 by Harms and Rolinger [5]. During the same era, the anatomical structure known as Kambin's triangle was introduced [6]. Over the subsequent 2 decades, minimal invasive transforaminal LIF (MIS-TLIF) and full-endoscopic transforaminal lumbar interbody fusion (Endo-TLIF) have evolved. MIS-TLIF offers advantages such as reduced soft tissue damage, minimized muscle retraction, reduced intraoperative blood loss, and shorter hospitalization times compared to open surgery [7]. Endo-TLIF, on the other hand, provides superior short-term clinical outcomes, faster postoperative recovery, less blood loss, shorter hospital stays, and reduced muscle retraction compared to MIS-TLIF, with similar long-term clinical outcomes, fusion rates, or complication rates [8-10]. While Endo-TLIF has gained popularity, it is not without its challenges, including a steep learning curve, extended surgical time, and the risk of nerve root injury (NRI) [11,12]. Despite these drawbacks, its excellent clinical outcomes have contributed to its increasing use in recent years [13].
Currently, 2 common approaches to full-endoscopic lumbar fusion surgery are employed: full-endoscopic facet-sparing TLIF (FE fs-TLIF) and full-endoscopic facet-resecting TLIF (FE fr-TLIF) [14-16]. In FE fs-TLIF, also called KLIF (trans-Kambin lumbar interbody fusion) named by Ishihama et al. [14], a limited foraminoplasty is performed from the ventral aspect of the inferior vertebra’s superior articular process (SAP) to the SAP-pedicle junction. A working cannula is then inserted into Kambin triangle, defined by the exiting nerve root anteriorly, the inferior vertebra's endplate inferiorly, and the facet joint posteriorly [17]. Following complete discectomy, the bone graft and cage are introduced. Conversely, FE fr-TLIF involves resecting the ipsilateral inferior articular process (IAP) and SAP, with the working cannula inserted through an extended Kambin triangle. Discectomy is completed, followed by the insertion of the bone graft and cage. These 2 approaches share common complications associated with endoscopic spinal procedures, such as postoperative headache, neck pain, or postoperative hematoma [18-20]. However, due to distinct surgical routes, FE fs-TLIF is associated with a higher incidence of exiting NRI (ENRI), while FE fr-TLIF is linked to increased traversing NRI and incidental durotomy compared to FE fs-TLIF [21-25]. Given the risk of NRI, emphasizing nerve root protection is crucial due to potential prolonged hospital stays, increased medical costs, and compromised patient-reported outcomes [16,19,26,27].
Recognizing the significance of nerve root protection, this manuscript will provide a narrative review of surgical techniques, operative corridors, incidence rates, clinical manifestations, management strategies for NRI, and current methods for nerve root protection.
DETAILED SURGICAL TECHNIQUES OF FE fs-TLIF AND FE fr-TLIF
1. Surgical Techniques of FE fs-TLIF
Anesthesia, comprising general, local, or epidural modalities, is administered preoperatively based on established protocols [19,28]. Following anesthesia, the patient is positioned prone, and precise localization of the target vertebral level is marked. Subsequently, a meticulous sterile preparation is executed. The surgical incision is situated 40–80 mm lateral to the spinal midline depending on the individual anatomical variations and surgeon’s preference, with the choice of the side guided by the patient's specific clinical symptoms. Following the incision, a skillfully placed endoscopic working cannula is introduced, with precise fluoroscopic guidance, and accurately positioned over the SAP. The ventral aspect of the SAP is fastidiously resected until it reaches the junction with the pedicle, thereby completing the foraminoplasty. The working cannula is further advanced into Kambin triangle to facilitate the discectomy and endplate preparation. Ultimately, after trial testing for size and positioning, a cage glider is introduced to establish a secure space, culminating in the definitive cage insertion under fluoroscopic guidance [15,16,24].
2. Surgical Techniques of FE fr-TLIF
Analogous to FE fs-TLIF, FE fr-TLIF necessitates the administration of general or epidural anesthesia in the preoperative phase. A notable distinction is the infeasibility of conducting FE fr-TLIF under local anesthesia [19]. Following anesthesia induction, the patient is positioned in a prone orientation using a radiolucent spinal operating table. Precise localization of the target vertebral level is meticulously executed before surgery. After comprehensive sterile preparation, a fluoroscopic-guided relocalization is performed immediately before the incision. Typically, the incision is made over the lateral pedicle line of cranial pedicle, with the choice of the side guided by the patient's predominant clinical symptoms. Subsequent to the incision, a working channel is inserted and docked onto the pars interarticularis of the upper vertebra. Utilizing endoscopic visualization, the medial and lateral margins of the IAP are exposed. Initially, the resection of the ipsilateral IAP is performed, either from the spinolaminar junction to the superolateral region using an inside-out technique, or vice versa, employing an outside-in technique. Subsequently, resection of the ipsilateral SAP of the caudal vertebrae is also performed, extending from the junction of SAP and the superior lamina of the inferior vertebra to the junction of SAP and the transverse process. The working cannula is then advanced into the extended Kambin's triangle to facilitate discectomy and endplate preparation. Finally, a cage glider is introduced to establish a secure quadrangular space, and the final cage is inserted following a trial test to ensure the appropriate size and positioning under fluoroscopic guidance [15,16,24].
3. Variations in Surgical Procedures between FE fs-TLIF and FE fr-TLIF
Several key distinctions exist in the operative techniques employed in FE fs-TLIF and FE fr-TLIF. These differences are pivotal for clinical decision-making. The primary differentiation lies in the choice of anesthesia, as FE fs-TLIF may be conducted under local anesthesia, while FE fr-TLIF usually necessitates general anesthesia [19]. Furthermore, the location of the skin incision in FE fs-TLIF is notably more lateral to the midline compared to FE fr-TLIF. In FE fs-TLIF, the expansion of the surgical space involves ventral facetectomy, specifically foraminoplasty or foraminotomy. In contrast, FE fr-TLIF entails complete facetectomy, including the resection of both the IAP and the SAP. The surgical access in FE fs-TLIF is denoted as Kambin triangle. On the other hand, the surgical corridor in FE fr-TLIF is referred to as the extended Kambin triangle, characterized by the convergence of Kambin's triangle and the interlaminar space [19]. Lastly, in FE fs-TLIF, nerve decompression is achieved primarily through techniques such as disc height restoration with interbody fusion and stabilization of dynamic instability. In contrast, FE fr-TLIF permits direct decompression of neural structures through the removal of hypertrophied ligamentum flavum or facet joint spurs. It may even encompass the completion of a unilateral laminotomy with bilateral decompression [29].
4. The restricted operative corridor of FE fs-TLIF and FE fr-TLIF
According to the existing literature, FE-TLIF is characterized as a LIF approach utilizing endoscopic assistance through the intervertebral foramen, specifically employing the anatomic corridor known as Kambin’s triangle. This triangular space is demarcated by the exiting nerve root, facet joint, and the superior endplate of the inferior vertebrae [6]. In a cadaveric study conducted by Min et al., the mean distance from the nerve root to the SAP of the inferior vertebrae was found to be 11.6±4.6 mm, displaying considerable variability. The study underscores the importance of executing discectomy procedures under direct visualization to avoid blind puncture due to the narrow safe zone [30]. Similarly, Hoshide et al. [31]'s cadaveric analysis indicated Kambin's triangle length ranging from 10 to 18 mm, gradually decreasing from L5 to L1 levels. Nagamatsu et al. [17]'s 3-dimensional image analytic study illustrated a gradual decrease in the angle of the exiting nerve root to thecal sac from L2 to S1. The combination of limited distance from ENR to SAP and diminishing nerve root angle exposes the procedure to the high risk of direct root stabbing or compression. A machine-assisted 3D computed tomography/magnetic resonance imaging (CT/MRI) fusion imaging study also demonstrated the restricted distance from ENR to SAP, ranging from 3.79 to 5.82 mm, highlighting the vulnerability of the ENR during endoscopic surgery [32]. While Kirschner wire and needle insertion are relatively safe in the confined anatomical space of Kambin triangle, the success rate of a 5-mm dilater passage without root irritation only ranges from 8.7% to 50.0% [30,31]. Given the heightened risk of nerve root irritation in the restricted natural anatomical corridor of the transforaminal approach in endoscopic surgery noticed in cadaveric and imaging analysis, there is a recognized necessity for a procedure to expand the surgical corridor of Kambin’s triangle. This expansion is crucial to enhancing the safety and efficacy of FE-TLIF procedures.
In FE fs-TLIF, a retrospective study reported a high operative failure rate of 10.3% when foraminoplasty was absent [33]. The finding suggests that the absence of foraminoplasty pose challenges in accessing the disc space and limit the space for inserting the working cannula, leading to incomplete removal of herniated disc material or insufficient nerve decompression [34]. Addressing this challenge, Sairyo et al. conducted a cadaveric study demonstrating transforaminal ventral facetectomy during endoscopic surgery. Postoperative computed tomography scans clearly showed an enlarged intervertebral foramen, facilitating the safe insertion of a working channel [35,36]. In clinical research, the consistent recommendation of foraminoplasty aims to expand the surgical area by resecting the ventral part of the SAP, thereby reducing nerve root irritation [37-41]. This evidence underscores the critical role of foraminoplasty in FE fs-TLIF, not only in improving the surgical success rate but also in ensuring comprehensive disc space access and the safety of exiting nerve root.
In the evolutionary trajectory of FE fs-TLIF, Jacquot and Gastambide [42] reported a frequent postoperative complication with an incidence of 36% in 2013. This high incidence is hypothesized to be related to the limited surgical access of Kambin triangle. To overcome these limitations and reduce postoperative complications, surgeons adopted FE fr-TLIF. This approach involves complete facetectomy, akin to open TLIF and minimally invasive TLIF [43,44]. Through complete facetectomy, the confluence of the neuroforamen and interlaminar space creates the extended Kambin triangle [45]. Although there is currently no cadaveric or imaging study precisely illustrating the extent of surgical corridor expansion after facetectomy in endoscopic spinal surgery, the technique is postulated to provide a substantial increase in the surgical access area. This enlarged anatomical space allows for the insertion of larger cages, mitigating the risk of over-retraction or compression of the surrounded nerve root [19].
INCIDENCE AND POSSIBLE CAUSES OF NRI
Neurological safety in endoscopic spinal operations plays a pivotal role, primarily attributed to the profound impact of NRI on postoperative clinical outcomes. Lewandrowski et al. [46] provided compelling evidence by demonstrating a direct correlation between postoperative dysesthesia and poor long-term functional outcomes. In FE fs-TLIF, the surgical corridor is in proximity to the exiting nerve root, leading to the typical complication of ENRI. Sairyo et al. reported an incidence of ENRI ranging from 1.0% to 8.9% and proposed 2 possible mechanisms: direct stabbing injury and compression of the nerve root during cannula insertion due to limited operative space [26,27,42,47-49]. Notably, dorsal root ganglion irritation was also documented as a significant contributor to postoperative dysesthesia [50]. Conversely, during FE fr-TLIF, the surgical access and cage entry point are closer to the thecal sac and traversing nerve root, resulting in common complications such as traversing NRI, incidental dural tears, or epidural hematoma [16]. Kim et al. [15,16] reported the incidence of traversing NRI presenting with transient paresthesia ranges from 1.0% to 3.0% due to nerve root irritation. The occurrence of traversing root injury is frequently associated with the use of instruments like the Kerrison punch, power burr, or disc clamp [51,52].
CLINICAL MANIFESTATION OF NRI IN ENDOSCOPIC SPINAL SURGERY
Given the elevated incidence and substantial clinical repercussions of NRI, it is imperative for endoscopic spine surgeons to possess a thorough understanding of its clinical manifestations. Drawing from the literature, the spectrum of clinical presentations associated with NRI encompasses radicular pain, limb paresthesia, dysesthesia, muscle weakness, limb numbness, or even drop foot [22,42,53-56]. Sairyo et al. [49,57] delineated that symptoms of direct stabbing injury to the nerve root during cannula insertion typically manifest immediately after the operation. Conversely, symptoms related to NRI due to compression by surgical instrumentation, linked to limited anatomical space, often present days after the endoscopic surgery. The hypothesized mechanisms include transient neurapraxia resulting from stabbing injury and ischemic reperfusion injury upon the removal of compression from the nerve root by the working channel [26,58,59].
Despite the imperative of early NRI detection, differential diagnosis should encompass rebound pain or numbness postoperation, postoperative epidural hematoma, incomplete decompression, recurrent herniated disc or vertebral infection [18,60]. Lin et al. [61] in 2022 elucidated a 5.8% incidence of rebound pain or numbness typically occurring within 2 weeks, of lower severity compared to preoperative status, and spontaneously resolving with symptomatic management. Distinguishing disease recurrence, which is more severe and usually requires reoperative treatment, is crucial and typically occurs within 3 months [61,62]. Postoperative spinal epidural hematoma, with symptoms like severe surgical site pain, radiculopathy, decreased muscle power, or bladder dysfunction, more frequently presents within 24 hours, especially between 4 to 6 hours [63,64]. These nuances must be considered alongside NRI for a comprehensive diagnostic approach.
MANAGEMENT OF NRI IN ENDOSCOPIC SPINAL SURGERY
Clinical symptoms associated with NRI typically exhibit a tendency to spontaneously resolve with conservative treatment. While no study has definitively outlined the efficacy of pharmacological treatments for NRI postendoscopic spinal surgery, the use of acetaminophen, nonsteroidal anti-inflammatory drugs, opioids, and pregabalin may be considered as appropriate options [57]. Additionally, recommendations include prolonged bed rest and the use of a back brace for support [65]. In cases where conservative treatment proves ineffective, or there is evidence of disease recurrence or postoperative hematoma, surgical intervention becomes a viable consideration [60-62]. Surgical management may involve addressing persistent or recurrent compression of the nerve root, thereby aiming to alleviate symptoms and enhance the overall outcome for the patient.
THE PREVENTIVE STRATEGIES OF NRI IN ENDOSCOPIC SPINAL SURGERY
1. Preoperation
1) Well-designed training program
Given the steep learning curve associated with endoscopic spinal surgery, a meticulously designed training program is recommended to reduce intraoperative complications [66]. Training opportunities, such as medical conferences, workshops, and international meetings, have proven effective in influencing spine surgeons' clinical practices for managing degenerative lumbar diseases [51]. The incidence of intraoperative complications is believed to have a negative correlation with the surgeon's technique and experience [57]. Excluding outlier data from certain immature surgeons can significantly shift the complication rate from 1.07% to 0.32% [51,67]. In summary, a comprehensive training program is essential to eliminate the risk of NRI and enhance the surgical technique of spinal surgeons.
2) Complete preoperative imaging evaluation
The restricted anatomical space in spine endoscopic surgery is an ongoing challenge for surgeons, as an excessively limited surgical corridor may elevate the risk of NRI or compression [49]. In certain patients, the adequate Kambin triangle might be absent, particularly at specific instrumented angles and lumbar levels [17]. Therefore, a thorough preoperative imaging evaluation is necessary to decide the endoscopic approach, utilizing computed tomography or magnetic resonance imaging to reduce operative complications [26]. The use of 3D CT/MRI is also recommended for its ability to simultaneously evaluate soft tissue and bony structures [17].
2. Intraoperation
1) Precision in local anesthesia during endoscopic spina operation
In the peri-operative phase of FE-TLIF surgery using the facet-sparing approach, the meticulous administration of precise local anesthesia is crucial to reduce the risk of ENRI [19,57,68]. It is essential to execute precise local anesthesia while avoiding infiltration of the exiting nerve root, as this could potentially mask the symptoms of NRI [57]. Tai et al. [37] recommend a specific technique involving the administration of 3 to 5 mL of 1% lidocaine at the subcutaneous and fascia layers, followed by 5 to 10 mL of 1% lidocaine infiltration from the muscle layer to the junction of the pedicle and SAP. This injection route minimizes the risk of nerve block of the exiting root. Finally, the needle is inserted into the ventral SAP, and 5 to 10 mL of 0.5% lidocaine is administered to block the sensation of the facet joint [37]. By employing appropriate local anesthesia, patients can detect any dysesthesia or paresthesia sensations during the operation, allowing for immediate intervention, such as stopping the procedure or adjusting the surgical cannula, to help reduce the incidence of injury to the exiting nerve root [35,69]. Despite suggestions from multiple authors that endoscopic fusion surgery under local anesthesia decreases the incidence of NRI, there is currently no head-to-head research comparing the operation with or without local anesthesia regarding the incidence of NRI.
2) Intraoperative neurophysiological monitoring
The implementation of intraoperative neurophysiological monitoring has been widely discussed as a preventive measure against nerve damage. Various neuromonitoring tools, including motor-evoked potential, electromyography, and somatosensory-evoked potential, have been listed. In transforaminal endoscopic discectomy, patients monitored with sensory-evoked and transcranial motor-evoked potential have demonstrated a reduced incidence of postoperative dysesthesia through the repositioning of surgical instrumentation [70]. Studies by Nagahama et al. [71] and Abbasi et al. [72] have highlighted the benefits of neural monitoring using somatosensory-evoked potential and electromyography, respectively. Recognized as a crucial method to enhance neurological safety in spinal fusion surgery, an increasing number of endoscopic spinal surgeons are advocating for the incorporation of neuromonitoring during operations to promptly readjust surgical instrumentation and diminish the risk of nerve root irritation [70,73-76].
3) Surgical technique
(1) The inside-out and outside-in technique for IAP resection
In FE fr-TLIF, highlighting the importance of facet joint resection to establish a surgical corridor is emphasized for procedural facilitation [16,24]. For the surgical manipulation of the IAP, specific anatomical locations were defined by Kim's point and Wu's point. Kim’s point is identified as the intersection of the SAP tip and IAP, while Wu’s point is defined as the junction of the cranial vertebral lamina to IAP [16,24,77]. In 2021, Kim et al. [15] elucidated the differences between the inside-out and outside-in techniques for IAP resection. The former involves resection from Wu’s point to Kim’s point, whereas the latter is performed from Kim’s to Wu’s point. The results indicated a lower operative time in the outside-in group. This outcome was attributed to reduced paraspinal muscle dissection, limited intraoperative bleeding, and an improved clear surgical field, thereby avoiding nerve injury. Additionally, decreasing the operative time to hemostasis contributes to minimizing the duration of compression on the nerve root.
(2) Gentle surgical technique of working cannula insertion
Given the proximity of the nerve root, it is imperative to employ a gentle approach during working cannula insertion. Soo et al. [78] introduced the rotate-to-retract technique in full-endoscopic facet-resecting lumbar decompression, involving the initial insertion of the one-tip working cannula's opening bevel surface on the cranial side, followed by rotation to the caudal side to prevent stabbing injury to the nerve root. Cho et al. [79] described a floating technique, initiating the insertion of the guidewire along the lateral margin of the superior one-third of the SAP of the lower vertebrae, progressing to the superomedial border of the lower pedicle. The tip of the cannula is then tilted to the cranial side with compression of perineural tissue. Summarizing previous literature, safe working cannula insertion is crucial for reducing the risk of NRI, involving steps such as (1) inserting the open bevel of the cannula facing the nerve root and slowly rotating it to retract the nerve root, and (2) docking the guidewire and cannula over the base of the superior vertebral notch of the lower vertebrae initially to avoid direct injury to the nerve root during instrument insertion.
(3) Adequate foraminoplasty
During the FE fs-TLIF, foraminoplasty is performed through the resection of the ventral SAP to create a larger surgical corridor, preventing postoperative dysesthesia. Lee et al. [80] emphasized the importance of foraminoplasty in averting direct injury to the exiting nerve root during instrument insertion. The expansion of the neural foramen aids in avoiding nerve root compression during the procedure [81-83]. Foraminoplasty is recommended to commence from the base of the SAP rather than the tip to decrease root irritation [84]. Various surgical tools for foraminoplasty, including high-speed burrs, trephines, reamers, or laser-assisted methods, have been mentioned [41,85-87]. In 2018, Yang et al. [84] recommended trephines and reamers over burrs and lasers, citing reduced thermal damage.
4) Cage glider design
During endoscopic procedures, a specially designed cage glider has been introduced to mitigate the risk of NRI and enhance neural tissue protection. Kim et al. [16] recommended the Harrison cage glider for use in the FE fr-TLIF, featuring 2 long tips that simultaneously protect the exiting and traversing nerve roots. In a comparative context, Sairyo [49] elucidated the use of a single-tip, oblique bevel cage glider during the FE fs-TLIF, effectively shielding the exiting nerve root from injury. Both special-designed glider should be placed with safe steps and technique just like above mentioned retraction and rotation technique.
5) The control of operative time
Sensory nerve fibers are susceptible to compressive forces, leading to nerve dysfunction and dysesthesia [88]. Considering the contribution of NRI resulting from the compression of the working cannula during the operation, reducing operative time becomes a method to diminish prolonged irritation of the nerve root. Choi et al. [26] conducted an analysis of 20 patients undergoing transforaminal endoscopic discectomy and identified prolonged surgical time as a risk factor for NRI. Moreover, due to the persistent pressure of irrigation fluid during endoscopic surgery, reducing surgical time also plays a role in lowering nerve irritation associated with increased epidural pressure [18]. In summary, optimizing surgical time stands as an effective method to safeguard nerve tissues.
CONCLUSION
In conclusion, prioritizing meticulous surgical techniques, comprehensive training, and preventive measures such as precise local anesthesia and neuromonitoring is paramount in minimizing nerve root injuries during endoscopic spinal surgeries. These strategies collectively contribute to enhanced patient safety and positive postoperative outcomes in this specialized field.
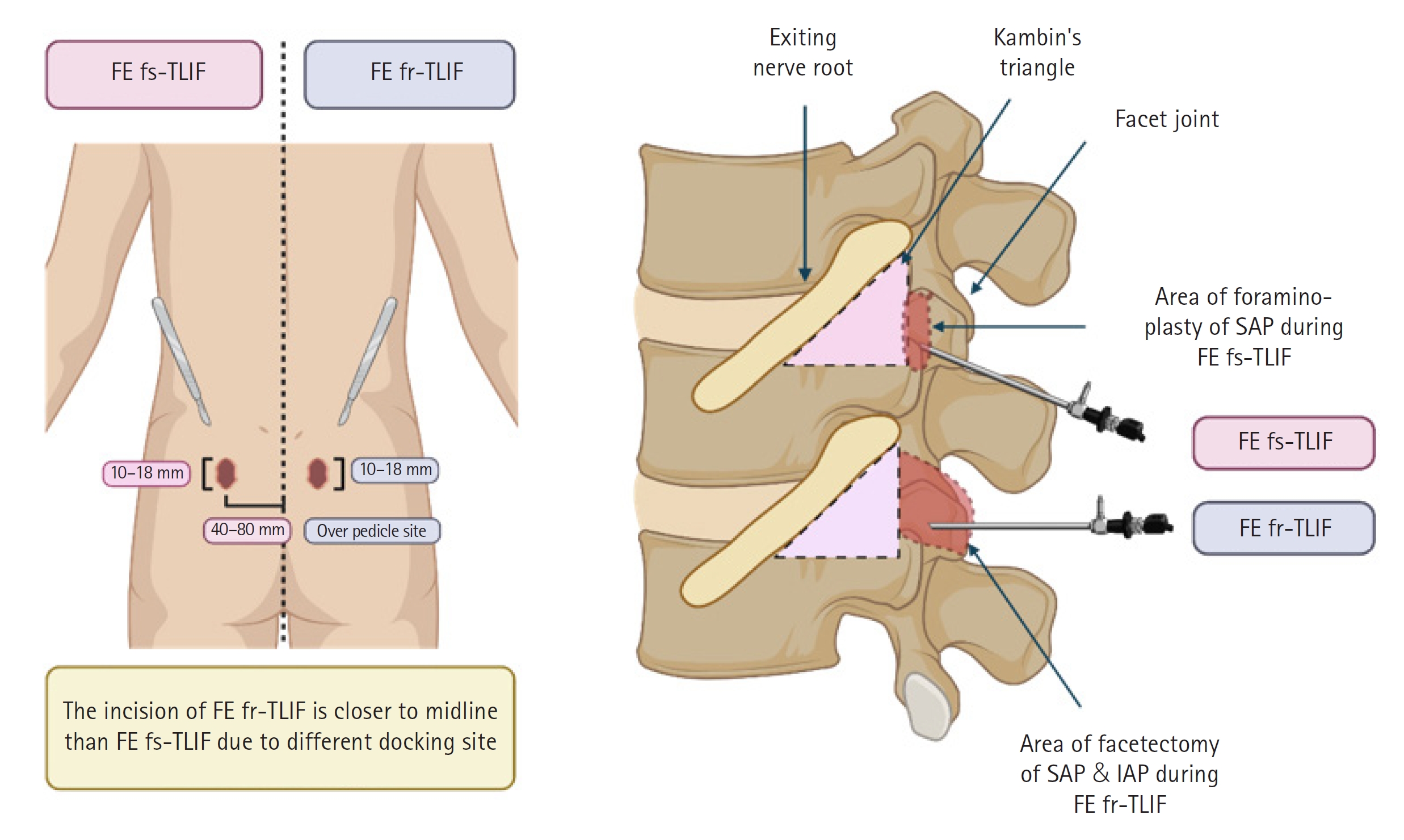
Graphical Illustration of FE fs-TLIF and FE fr-TLIF. The left portion of the figure illustrates the disparity in surgical incision sites between FE fs-TLIF and FE fr-TLIF. In FE fs-TLIF, the incision is made 40 to 80 mm lateral to the midline, while in FE fr-TLIF, it is positioned closer to the midline and precisely over the pedicle site, as confirmed with fluoroscopy. The right portion of the figure outlines the surgical routes for FE fs-TLIF and FE fr-TLIF. In FE fs-TLIF, following foraminoplasty, the working cannula is inserted into Kambin triangle to facilitate subsequent discectomy. In contrast, in FE fr-TLIF, after complete facetectomy, the surgical corridor of the extended Kambin triangle is accessed, and the working cannula is inserted. The red dotted line delineates the area where bony structures are surgically removed during the procedure, while the pink dotted line demarcates Kambin triangle. TLIF; transforaminal lumbar interbody fusion; FE fs-TLIF, full-endoscopic facet-sparing TLIF; FE fr-TLIF, full-endoscopic facet-resecting; SAP, superior articular process; IAP, inferior articular process.
Notes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study was financially supported by grants from the Ministry of Science and Technology, Taiwan (MOST 110-2314-B-006 -027 -MY3 & NSTC 112-2314-B-006-075-) awarded to C.L.L.