Full-Endoscopic Discectomy and Debridement for Iatrogenic Spondylodiscitis After a Lumbar Peritoneal Shunt
Article information
Abstract
Lumbar peritoneal shunt (LPS) is the standard treatment for nonobstructive hydrocephalus. Shunt infection, overdrainage, bleeding, and cerebrospinal fluid leaks have been reported as LPS complications. We present a 70-year-old man who developed iatrogenic spondylodiscitis 2 weeks after LPS placement, experiencing severe back pain and neurological deficits. Despite the empiric antibiotics, his symptoms persisted. The patient underwent fully endoscopic debridement and drainage (FEDD) to address the infection without LPS removal. After the procedure, the patient experienced a significant reduction in pain. Even though pathogen cultures were negative, the empiric antibiotic treatment continued for 6 full weeks. The patient was able to ambulate with a thoracolumbar orthosis due to the lumbar kyphotic deformity. FEDD, in conjunction with effective antibiotics, offers rapid pain relief and functional improvement in iatrogenic spondylodiscitis, even with LPS placement. However, FEDD may not correct spinal deformities and is unsuitable for advanced spinal disease or instability. Early detection of spondylodiscitis is crucial for improved outcomes.
INTRODUCTION
Lumbar peritoneal shunt (LPS) is an effective and commonly seen treatment for nonobstructive hydrocephalus [1,2]. There are reported complications such as shunt infection, overdrainage, bleeding, and cerebrospinal fluid (CSF) leak [2]. However, iatrogenic spondylodiscitis after LPS has never been reported as a complication of LPS.
Iatrogenic spondylodiscitis is rare but fatal where it involves the infection and inflammation of the intervertebral discs and adjacent vertebrae after lumbar surgery or following invasive diagnostic or therapeutic procedures [3-5]. The diagnosis of spinal infections is often challenging due to their insidious onset with nonspecific signs and symptoms [6]. The diagnosis usually depends on the patient’s symptoms and signs, imaging studies such as x-rays and magnetic resonance imaging (MRI), as well as laboratory tests like erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) [6]. A computed tomography (CT)-guided biopsy helps for establishing a bacterial diagnosis, and it allows tailoring antibiotic treatment [6,7]. However, studies suggest that the positive rate for culture is relatively low, and the outcomes for both culture-positive and culture-negative cases were similar in terms of antibiotic treatment [7].
It tends to occur in a population such as elderly and immunocompromised patients, and it is due to hematogenous spread of infection from other organs or direct extension of infection following surgery, lumbar puncture, trauma, or local infection [4,5].
Conservative treatment with antibiotics and bed rest could be sufficient for mild infections [8,9]. A surgical procedure could be indicated when advanced bone destruction, progressive deformity, severe neurological deficit, or progressive infection occur [10]. LPS removal is also considered if there is a shunt infection or if the infection progresses [11]. Here, we present a 70-year-old male with iatrogenic spondylodiscitis after LPS placement. The patient underwent empiric antibiotic therapy for 3 weeks and underwent full-endoscopic debridement and drainage (FEDD) without removing the LPS due to sustainable back pain with neurological deficits.
CASE REPORT
A 70-year-old male patient with a history of prostate adenocarcinoma treated with radiotherapy also has Parkinson's disease, managed with oral medication. This time, he was newly diagnosed as normal pressure hydrocephalus (NPH) 6 months ago from the symptoms, brain MRI, and the CSF tap test [12]. This time, he went through LPS placement due to diagnosed NPH. Two weeks later, the patient developed progressive acute low back pain with a throbbing quality of 8 out of 10 on the pain scale, worsened by spine extension and flexion. He also complained of bilateral leg weakness, mostly severe over extension of the right thigh with numbness over right L4 dermatome, leading to difficulty ambulating.
A lumbar MRI confirmed the presence of acute inflammatory changes, edema, and annulus ruptures in the L3–4 disc, which were primarily compressing the right L3–4 lateral recess. The spinal catheter for LPS could be notified just at the L3–4 level (Figure 1A and B). The initial white blood cell (WBC) count, ESR, and CRP were within the normal range. However, the lumbar MRI suggests ongoing inflammatory processes suspected for spondylodiscitis. A CT-guided biopsy for bacteriological culture was suggested, but the patient refused. Since then, empiric antibiotic treatment has been initiated. Three weeks later, WBC count, CRP, and ESR had elevated with persistent severe back pain and the right leg pain. He exhibited no symptoms or signs indicative of meningitis, pneumonia, urinary tract infection, infective endocarditis, or surgical site infections. Pathogens were not detected in the blood, sputum, urine, or during tapping of the LPS shunt. CSF analysis from the shunt tapping showed no signs of infection. Considering the spinal catheter at the level of L3–4 through which the puncture was made, iatrogenic spondylodiscitis without LPS infection was considered. Subsequently, after discussing with the patient, FEDD was arranged, aiming for improving his back pain and the neurological function.
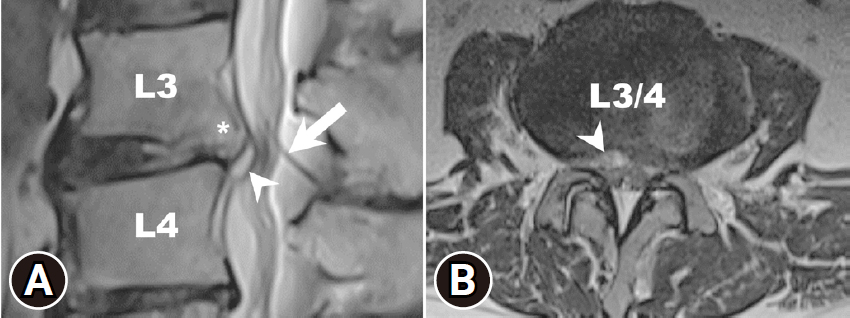
Lumbar magnetic resonance imaging (MRI) was performed 2 weeks after lumbar peritoneal shunt (LPS) placement. (A) Sagittal T2-weighted MRI shows acute inflammatory changes with edema (arrowhead) and annulus ruptures of the L3/L4 disc (star), with the visible spinal catheter of LPS (arrow) situated at the infection site. (B) Axial T2-weighted MRI image shows the L3/4 intervertebral disc compressing the right lateral recess (arrowhead).
SURGICAL PROCEDURE
1.Patient Positioning and Skin Marking
The procedure was performed under local anesthesia and the patient was aware of each step of the procedure. After he was placed in a prone position on the radiolucent table, the entry point was determined to be about 10 cm from the midline. The trajectory line towards the base of the superior articular process (SAP) was drawn on the skin, obtained from anterior-posterior (AP) and lateral fluoroscopy.
2. Needle Puncturing and Working Cannula Docking
After determination of the entry point, an 8-mm stab incision is made through the skin and the fascia using the No. 15 blade after injecting 1% lidocaine subcutaneously. Subsequently, a cannulated needle is inserted from the entry point. 1% lidocaine was infiltrated into the muscle and the fascia along the trajectory towards the SAP. Following the tip of the cannulated needle docked on the base of the SAP confirmed by AP and lateral fluoroscopy, 0.25% diluted lidocaine was infiltrated to avoid excessive pain from the sequential manipulation [13]. A guide wire was then introduced through the cannulated needle, the sequential dilator was used to create the track for the working cannula. The working cannula was introduced and the tip of the working channel was docked at the base of SAP. After it’s confirmed by the fluoroscopy, the working channel was rotated 90° gently to retract the exiting nerve away.
3. Full-Endoscopic Debridement and Drainage
The endoscope with 4.3-mm working channel (SPINENDOS GmbH, Munich, Germany) is introduced through the working channel, accompanied by continuous sterile saline irrigation. The radiofrequency coagulator (VANTAGE BIOTECH CO., LTD., Taoyuan, Taiwan) and grasping forceps were employed to dissect soft tissue under direct endoscopic visualization. Additionally, a high-speed diamond burr (SPINENDOS GmbH) is utilized to widen the working space in the foramen's ventral portion [14]. Once the position of the working cannula is adjusted into the epidural space, the inflamed disc and the granulation tissue with compressing the L4 traversing nerve was identified (Figure 2A and B), and attentive debridement was performed to remove the infectious disc and the granulation tissue. During the procedure, the radiofrequency bipolar probe was used to control hemostasis.
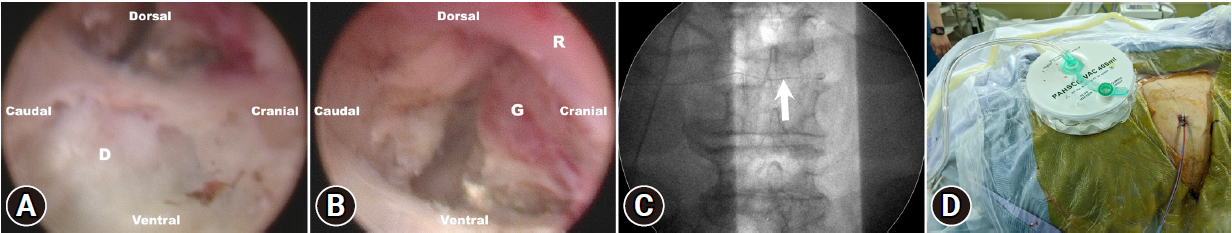
Vision under endoscopy. (A) Acutely inflamed annulus ruptures of the L3/L4 intervertebral disc are seen under endoscopic vision. (B) The epidural granulation tissue is compressing the traversing nerve root. (C) Placement of drainage (arrow) under fluoroscopic guidance. (D) The drainage is fixed on the back of the skin. D, intervertebral disc; G, epidural granulation tissue; R, traversing nerve root.
4. Final Check Point
As both the patient and the surgeon could mutually communicate during the procedure, the patient expressed the immediate right leg pain relief after removing the inflamed disc and the granulation tissue. After ensuring no other remaining pathology, we set the drain into the disc space and fixed on the back of the skin (Figure 2C and D). The surgical wound was closed with a single 3-0 nylon stitch.
5. Result
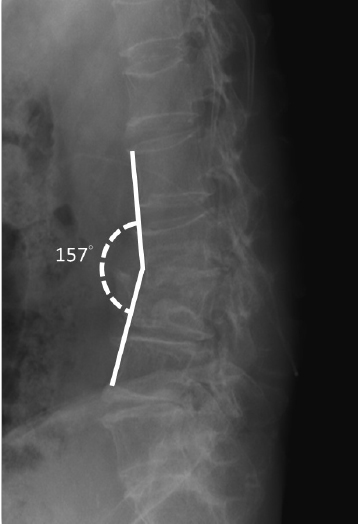
The patient experienced a significant reduction in back and leg pain. An MRI performed 3 days postoperatively showed decompression of the spinal canal at the level of L3–4 without compression of the right lateral recess (Figure 3A). However, focal irregular erosive changes are observed in the L3–4 spinal segment, resulting in a reduction in intervertebral space and apposition of the endplates (Figure 3B). The visual analogue scale (VAS) for back and bilateral leg pain decreased significantly from 10 to 5 immediately after the procedure in the sitting position (Figure 4C). The culture from the disc did not show any pathogen, the empiric antibiotic treatment was continued for the full 6-week course of the treatment. WBC count, CRP, and ESR showed significant reduction after the postoperative 1-week follow-up (Figure 4A and B). After 3 weeks postoperatively, the VAS score for the back and leg was 2 out of 10 (Figure 4C). The pathology report revealed neutrophilic infiltrate with fibrin exudate and granulation tissue in cartilage of the L3–4 disc (Figure 5A and B). Postoperative 6-month follow-up, the lumbar x-ray showed the lumbar kyphotic deformity (Figure 6). Ambulation was possible with the thoracolumbar orthosis.
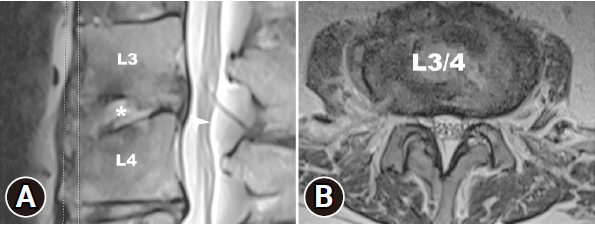
Postoperative 3-day lumbar magnetic resonance imaging (MRI). (A) Sagittal T2-weighted MRI shows decompression of the spinal canal, disappearance of annulus ruptures of the L3/4 disc, and acute inflammatory changes with edema (arrowhead) Irregular focal erosive changes (star) can be observed in the L3/4 spinal segment, leading to a reduction in the intervertebral space and the apposition of the endplates. (B) Axial T2-weighted MRI shows decompression of the right lateral recess.
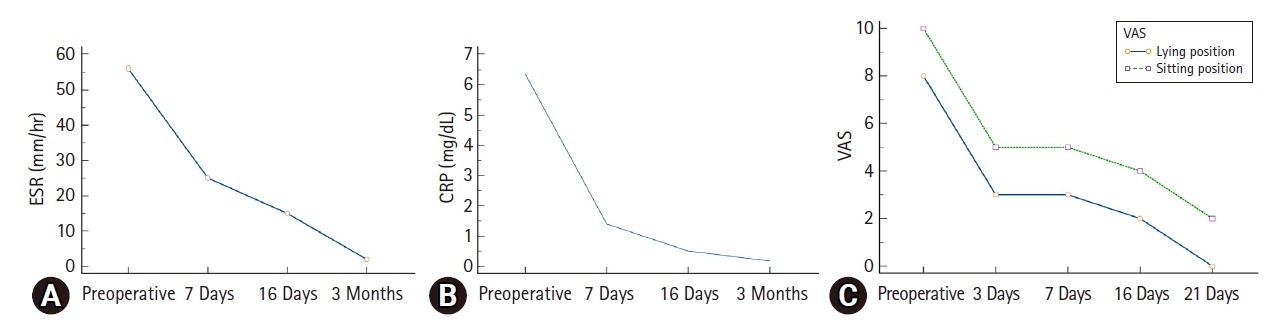
Laboratory data and the visual analogue scale (VAS) score of the patient. The erythrocyte sedimentation rate (ESR, A) and C-reactive protein (CRP, B) levels significantly decreased immediately after fully endoscopic debridement and drainage (FEDD). (C) The VAS score revealed that pain significantly improved right after FEDD.
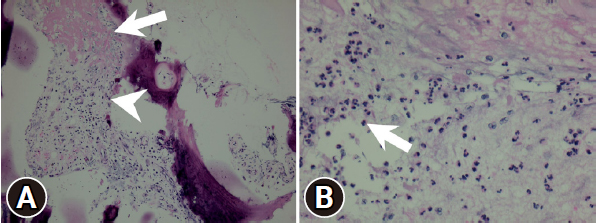
Pathology report of the intervertebral disc. (A) Neutrophilic infiltrate is seen in the cartilage (arrow) and in the intervertebral disc (arrowhead). (B) Epidural granulation tissue and mild neutrophilic infiltration with fibrin exudate (arrow).
DISCUSSION
LPS is a generally used modality to treat for the communicating hydrocephalus [1,2]. Although it’s known to be safe and effective, there are various kinds of complications reported such as malfunction, shunt infection, overdrainage, catheter migration, back pain, and radiculopathy [15,16]. Back pain has been reported up to 10% in the pediatric population, but it’s little reported in the adult population [16]. To our best knowledge, this is the first case reported iatrogenic spondylodiscitis with the formation of granulation tissue after LSP placement.
The diagnosis of spondylodiscitis would be delayed due to the insidious onset of the disease [17]. Once it’s diagnosed, early intervention is essential in the treatment of spondylodiscitis to prevent further damage to the spine [18]. Conservative therapy with antibiotics and pain management can help control the infection and reduce pain. An operative intervention is required if there is progressive neurological dysfunction, structural alignment, or infection even after the conservative measures [10].
In the present case, spondylodiscitis was observed in the lumbar MRI 2 weeks after the LPS placement, with no initial increase in WBC count, ESR, or CRP. A CT-guided biopsy for bacteriological culture was suggested to tailor antibiotic treatment [7]. However, considering the relatively high false-negative rates and the similar outcomes from antibiotic treatment, whether it is culture-positive or not [7], the patient hesitated. Despite empirical antibiotic treatment, the symptoms and signs persisted with elevated WBC counts, ESR, and CRP levels (Figure 4A and B). The increased values of these laboratory data support the idea of an ongoing infection in the spine. The pathogen detection from FEDD is relatively higher compared to CT-guided biopsy [10,19]. However, the absence of a detected pathogen in this case may be attributed to several factors: (1) Empiric antibiotic treatment administered before culturing the disc space, and (2) It could be a result of aseptic forms of spondylodiscitis, characterized by inflammation without any bacterial infection.
Pathologic reports showed neutrophilic infiltration with fibrin exudate and granulation tissue formation in the cartilage of the L3–4 disc supports the idea of ongoing bacterial infection and inflammation (Figure 5A and B).
Immunocompromised patients (diabetes mellitus, malignancy, and acquired immunodeficiency syndrome), local and remote infections, previous lumbar puncture, previous spine trauma, and lumbar catheterization are the predisposing factors which would stratify the risk of spondylodiscitis [5]. In this case, prostate adenocarcinoma treated with radiotherapy was the predisposing factor.
Iatrogenic spondylodiscitis may be due to hematogenous spread, extension of the local infection, or direct inoculation of the pathogen [5]. Direct inoculation of the pathogen occupies 25%–30% [17,20,21]. Since the patient had no other infection sources such as meningitis, infective endocarditis, pneumonia, urinary tract infection, or local infection. Considering spondylodiscitis occurred at the L3–4 level, where the spinal puncture was made and the spinal catheter was placed, direct inoculation from LPS placement is the most likely possibility. One reported that the rate of spondylodiscitis increases with multiple attempts of lumbar puncture and epidural hematoma after the puncture [4]. Similar to lumbar puncture, as in our case, multiple attempts at spinal puncture for placing the spinal catheter for LPS also increase the risk of infection [4,22].
FEDD is a minimally invasive surgical technique performed through a small incision, eliminating the need for the extensive tissue dissection and muscle retraction often associated with traditional open surgical techniques, which may lead to higher complication rates with severe comorbidities [10,22,23]. FEDD is a safe and effective procedure. However, its application for iatrogenic spondylodiscitis with a foreign body has not been previously described. As FEDD is done under continuous water irrigation, the pathogen could spread into the epidural space and infect the shunt system. No serious complications, such as meningitis or LPS infection, occurred following FEDD, primarily due to the constant maintenance of water flow-in and flow-out during the procedure. Additionally, continuous irrigation of the pathogen throughout the process resulted in the immediate resolution of spondylodiscitis, leading to a rapid reduction in back and leg pain. This facilitated ambulation with a thoracolumbar spinal orthosis without any progression of the infection. The effective empiric antibiotic treatment also contributed to controlling the infection.
FEDD has already been demonstrated efficacy in treating spondylodiscitis, exhibiting high rates of infection control and favorable patient outcomes [10]. Nevertheless, it is essential to acknowledge certain limitations. FEDD cannot correct spinal deformities like in this case. Additionally, it may not be suitable for patients with advanced spinal disease, or significant spinal instability [23-25].
CONCLUSION
For patients diagnosed with iatrogenic spondylodiscitis with LPS, FEDD with effective antibiotic treatment could provide rapid pain relief and improve functional status. It would also be a relatively low-risk procedure that is suitable for elderly or immunocompromised patients. FEDD could not correct spinal deformities. Therefore, early detection of spondylodiscitis would be crucial for a favorable prognosis.
Notes
Conflict of Interest
CMC, a member of the Editorial Board of Journal of Minimally Invasive Spine Surgery & Technique, is the author of this article. However, he played no role whatsoever in the editorial evaluation of this article or the decision to publish it. Author has no conflict of interest to declare.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.