INTRODUCTION
Lumbar spinal stenosis is a reduction in the volume of the central spinal canal, the lateral recesses, and/or neuroforamina that decreases the space available for the thecal sac and/or exiting nerve roots [1]. Yong-Hing and Kirkaldy-Willis [2] has well documented the cascade of events that leads to spinal stenosis. The combination of the ventral disk bulging, osteophyte formation, and the dorsal facet and ligamentum flavum hypertrophy combine to circumferentially narrow the spinal canal and the space available for the neural elements. This compression of the nerve roots of the cauda equina leads to the characteristic clinical signs and symptoms of lumbar spinal stenosis [3]. Lateral lumbar interbody fusion (LLIF) is a promising MIS surgery that can address this pathology by indirect decompression of the neural elements in an attempt to restore the native disc height through ligamentotaxis which stretches and tightens the remaining annular fibers, causes longitudinal distraction of the posterior longitudinal ligament, unbuckling of the flavum and consequent enlargement of the epidural space [4]. Among the different types of LLIF, Extreme Lateral Interbody Fusion (XLIF) is a frequently used procedure that is a true direct lateral approach to the lumbar spine passing through the retroperitoneum and the psoas muscle. However, the transpsoas approach is associated with access-related thigh pain caused by direct muscle injury with the added risk of injury to the lumbar plexus [5]. The use of neuromonitoring is mandatory during the procedure to avoid the risk of lumbar plexus injury because of the anatomical proximity to the lumbar plexus and the limited direct visualization [6]. Iliac crest at L4-5 may obstruct access to the L4-5 view level [7]. Oblique lateral interbody fusion (OLIF) was introduced initially by Michael Mayer in 1997 [8]. The mini-open OLIF procedure allows for psoas-preserving access to the lumbar spine via the anterior oblique retroperitoneal approach. OLIF has several potential advantages over LLIF, such as less invasion of the psoas muscle and lumbar plexus, direct visualization of sensory nerves and important structures such as the ureter and sympathetic trunk approximating the psoas muscle, no need for neuromonitoring, and consistent access to the L4-5 level in cases involving a high-riding pelvis [9].
Various studies are available regarding the efficacy of indirect decompression in degenerative lumbar canal stenosis but we found only one study which deals with its effectiveness in severe canal stenosis [9-12]. It is still not clear whether XLIF or OLIF with their indirect decompression technique is effective enough to not warrant a posterior direct decompression in severe lumbar central canal stenosis. There are many studies regarding the efficacy of OLIF/XLIF to cause fusion in spondylolisthesis and open up the central canal area but still, there are no significant studies that evaluate the necessity of an unplanned second posterior decompression in case of failure of indirect decompression to alleviate the preoperative neurological compression symptoms [13].
The aim of this study is to assess whether indirect decompression is sufficient in lumbar canal stenosis with Schizas grade [14] C and D and to study which patients require direct decompression. It also attempts to quantify the limitations of indirect decompression and attempts to detect which subset of stenosis is suitable for indirect decompression and which subset requires direct decompression.
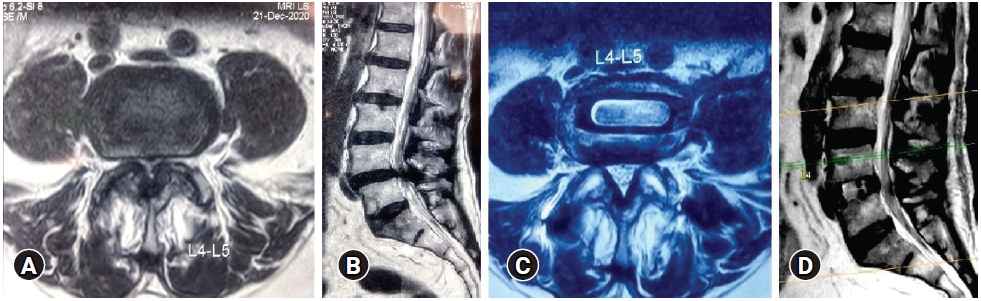
Description of the Schizas grading [14] is as follows:
Grade A stenosis: there is clearly CSF visible inside the dural sac, but its distribution is inhomogeneous
Grade B stenosis: the rootlets occupy the whole of the dural sac, but they can still be individualized. Some CSF is still present giving a grainy appearance to the sac.
Grade C stenosis: no rootlets can be recognized, the dural sac demonstrating a homogeneous gray signal with no CSF signal visible. There is epidural fat present posteriorly.
Grade D stenosis: in addition to no rootlets being recognizable there is no epidural fat posteriorly (Figure 1).
MATERIALS AND METHODS
We carried out an analysis of indirect decompression by distraction in 37 patients/44 segments that belonged to Schizas grade C and D following Schizas grading [14]. Informed consent was obtained from all the patients. All the patients were counseled for the requirement of direct decompression in case of persistent symptoms after OLIF.
Inclusion criteria: patients with neurogenic claudication with/ without back pain and rest pain and lumbar canal stenosis belonging to Schizas grade C and D with instability. Exclusion criteria: stenosis with instability belonging to Schizas grade A and B, stenosis without instability, trauma, infection, acute lumbar disc prolapse, patients with rest pain and listhesis >grade 3.
Clinical assessment of the patients was done by modified Macnab criteria (Table 1). All the surgeries were performed by a single surgeon and along with cage insertion; anterior or posterior fixation was carried in all the cases in the same stage. The approach in OLIF was from the left side in all the cases. The most proximal to distal levels for OLIF were L1-2 to L4-5. The demographics of the patients are demonstrated in the Table 2. Bone graft (autograft/ allograft+bonemarrow aspirate) was used in all the patients. An appropriate size cage was inserted through the oblique corridor under IITV guidance. No neuromonitoring was used.
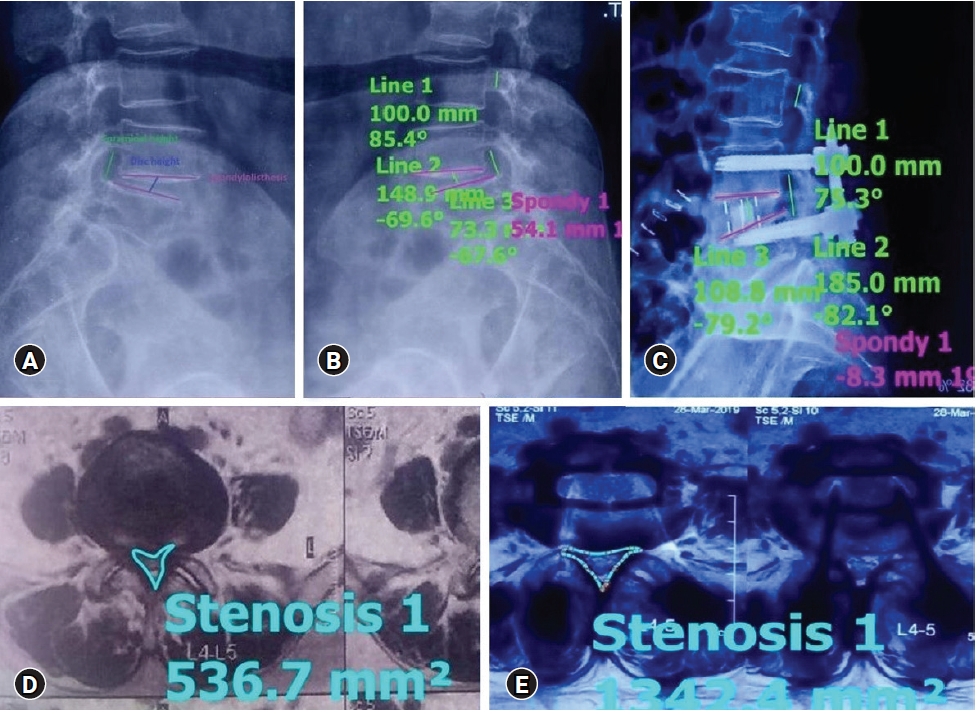
1. Assessment of Radiological Parameters (Figure 1)
Percentage improvement in disc height, foraminal height, segmental lordosis on X-rays, reduction of slippage of vertebrae, and increase in the overall area of spinal canal area were noted to assess indirect decompression.
1) Disc Height
Perpendicular from the midpoint of the cranial vertebral inferior endplate to the midpoint of the caudal vertebral superior endplate of the disc.
3) Foraminal Height
Distance between the cranial most and caudal most point of the foramen on lateral x-ray.
4) Spinal Canal Area
The spinal canal area was measured on MRI on a single axial slice through the center of the disc.
Pre-op and post-op comparisons of all these parameters were done and percentage improvement was calculated. Measurements were taken by two surgeons and an average of it was taken as the final measurement.
2. Statistical Analysis
Differences between the preoperative and postoperative variables were assessed using Wilcoxon signed Ranks test and paired T-test (continuous variables) and Chi-square test (categorical variables). A p-value <0.05 was considered statistically significant.
RESULTS
A total of 35 segments were studied on MRI. The rest of the segments could not be studied well because of the artifact effect. Case example of improvement in Schizas D type severe central canal stenosis after indirect decompression is shown in Figure 2.
2. Radiological Results (Table 3, 4)
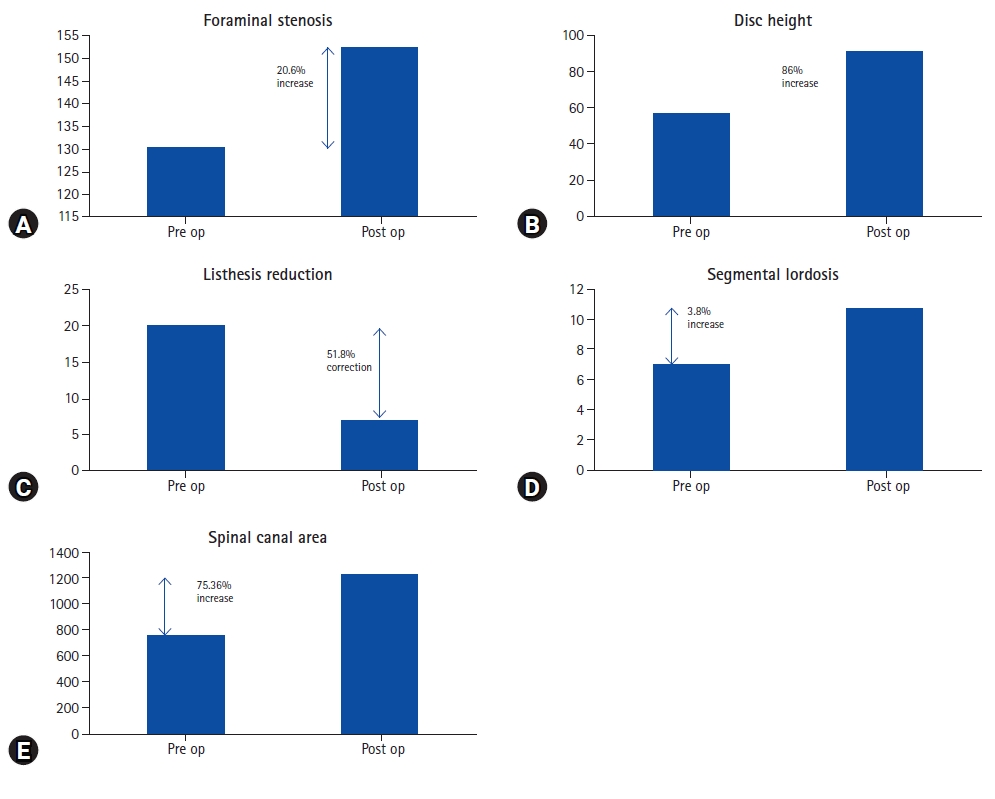
Improvement in different parameters from the pre-operative status is shown below in Figure 4.
3. Per Operative Complications
Intraoperative complications were seen in 3 patients (8%). 1 patient (2.7%) had intraoperative ALL rupture leading to increased instability which required anterior as well as posterior fixation. 1 patient (2.7%) had a peritoneal breach which was sutured immediately. 1 patient had a right L5 screw misplaced which caused immediate post-operative L5 radicular pain detected on CT scan and was revised the next post-op day.
4. Post-operative Complications
5 patients (13.51%) had immediate graft site pain which resolved in a period of 3–6 weeks. 2 patients (5.4%) had anterior thigh pain which resolved in 3–6 weeks. 1 patient (2.7%) had an incisional hernia but was nonprogressive and not causing trouble to the patient. 1 patient (2.7%) had significant subsidence at 1 month period following a jerk and had a recurrence of claudication pain. On MRI the stenosis at L4-5 level had reappeared and a posterior decompression surgery was required. Another notable complication was contralateral radiculopathy which was seen in 3 (8.1%) patients but recovered in 3–6 weeks. 2 patients (5.4%) had hip flexion weakness due to pain which recovered within 2 weeks. None of the patients had a ureteric or permanent neurological deficit. The overall rate of postoperative complications requiring attention was 8%.
DISCUSSION
The current study shows the efficacy of OLIF in achieving indirect decompression in severe spinal canal stenosis with Schizas grade C and D. There has been only a single study on indirect decompression in severe spinal canal stenosis i.e. Schizas grade C and D as severe central spinal canal stenosis has been considered to be a relative contra indication [4,12]. The role of indirect decompression in central canal stenosis has been addressed in various studies so far [11,15-18]. Our study included patients exclusively belonging to the Schizas grade C and D and who had neurogenic claudication. We had strict pre-operative selection criteria. None of the patients had rest pain in our study and due stress was given to the absence of rest pain in the supine position as preoperative assessment. None of the patients had any positive signs of nerve root tension. Patient having rest pain or positive nerve tension sign was excluded and considered not suitable for indirect decompression. We believe that rest pain and positive nerve tension sign are important clinical criteria requiring direct decompression. Khalsa et al. [19] showed in their study that rest pain have a significant association with reduction in Numeric Rating Scale (NRS) leg and back scores in patients undergoing indirect decompression for lumbar spinal stenosis.
None of our patients with Schizas grade C or D stenosis required direct posterior decompression though every patient was counseled for that if the symptoms do not subside. There have been various studies on indirect decompression but none of them but deals exclusively with severe canal stenosis hence it is difficult to compare our results with other studies. Lumbar canal stenosis has static and dynamic components. In our study, there is an increase in disc height by 86%, an increase in foraminal height by 20.6%, and listhesis reduction by 51%. All these have increased the overall spinal canal area by 75.36%. Spinal canal area of <75 mm2 is considered as severe lumbar canal stenosis [20]. Considering this criterion, in the study by Elowitz et al. [15], 9 patients fell in the category of severe canal stenosis <75 mm2, and the average increase in spinal canal area achieved in those patients was 262%. In our study, the increase in the overall spinal canal area was 75.36% which is less compared to Elowitz’s study [15]. Though it cannot be stated that all Schizas grade C and D always are <75 mm2 it does show that the increase in the spinal canal area (SCA) in patients with severe canal stenosis is significantly higher than in the patients with Schizas grade A and B [9,16,18,21]. There are wide variations in the measuring techniques of the SCA and that leads to a lot of variations in absolute values. Many attempts at developing algorithms for getting reproducible results are made [22]. Attempts at developing an algorithm for predicting success in indirect decompression are also made but they have not included severe canal stenosis in the study [23].
OLIF plays an important role in relieving the symptoms of neurological claudication not just by indirect decompression but also by providing stability [15]. This shows that the dynamic component of spinal stenosis has a major role to play in neurogenic claudication. Posture induces physiological changes in the CSA of the spinal canal and neural foramina in young asymptomatic volunteers as seen using MRI. At the disk level, the CSA of the spinal canal varied significantly depending on the body position, most notably between the upright flexed (mean, 268 mm2) and the upright extended (mean, 224 mm2) positions (p<0.0001). The maximum thickness of the ligamentum flavum was significantly increased in the extended positions (p<0.0001) [24]. Stabilization of the spine decreases the dynamicity of the segment. Hence both overall increase in the spinal canal area and decrease in the dynamicity are reflected in an improvement in the claudication symptoms of the patient by indirect decompression and no further need of direct decompression even in severe central canal stenosis.
The thickening of the ligamentum flavum is also thought to be due to the dynamic component of stenosis i.e. instability. The accumulation of mechanical stress, caused by age-related segmental instability, and especially segmental angulation with flexion-extension, leads to LF hypertrophy [25,26]. By doing indirect decompression and stabilizing the spine there is not only immediate effect on the increase in spinal canal area but in long term because of the effect of segmental stabilization there is remodeling of the ligamentum flavum and the overall spinal canal area further increases with time because of thinning of the ligamentum flavum. In a study by Ohtori et al. [10], it has been shown that the average CSA of the ligamentum flavum at the level of fusion 10 years after indirect decompression using ALIF was significantly less than that before surgery, and the CSA of the dural sac at the level of fusion was significantly larger than at other levels. Stability of the spine may have induced the change of the lumbar ligamentum flavum and remodeling of the spinal canal. It has been reported that following the immediate expansion of CSA due to disc height restoration, there is a gradual shrinkage of ligamentum flavum and reduction in disc bulging, further increasing the spinal canal area, which is in a significant proportion attributed to spinal stabilization and fusion advocating supplemental posterior percutaneous screw fixation [12]. The gradual shrinkage of ligamentum flavum and subsequent increase in the spinal canal area after OLIF is also established by the study of Mahatthanatrakul et al. [27].
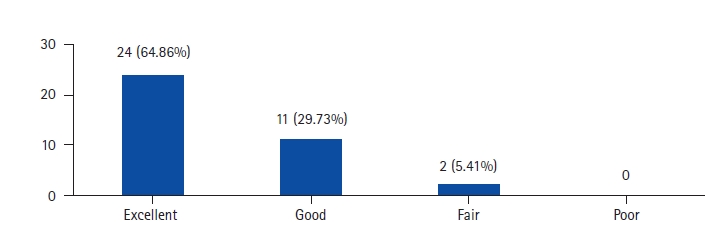
Radiographic results regarding indirect decompression of central canal stenosis are less consistent. In some studies, patients with severe spinal stenosis were excluded from the analysis [28]. Only mild increases of central canal area and relatively high rates of secondary posterior decompressions led Oliveira et al. [16] to conclude that the risk of failure for central canal stenosis decompression has to be emphasized during the patient consent process. Consequently, they concluded that central canal stenosis might be a relative contraindication in indirect decompression if patients seem to be incompliant regarding a potential additional laminectomy if symptoms persist. However, Elowitz et al. [15] found an improvement in clinical outcome scores even in patients with a modest increase in spinal canal area after indirect decompression, similar to our study, with 35 out of 37 patients showing good to excellent clinical results. This raises the question of how much decompression is truly required to alleviate the symptoms of spinal canal stenosis which points that there is likely a dynamic component to stenosis, as evidenced by the positional nature of the pain pattern. This dynamism may be further enhanced in hypermobile situations such as in degenerative spondylolisthesis. Indirect decompression works by first, increasing the absolute dimensions of the spinal canal and foramina through disc height restoration and slip reduction, but also by removing the dynamic component of the stenosis through the elimination of motion infusion. As such, despite no statistical change in the canal area, significant clinical improvements were realized and maintained [18]. Also in spondylolisthesis, the pseudobulge contributes to narrowing the canal and OLIF by distraction, improves the canal area by decreasing the disc bulge along with stretching and unbuckling of ligamentum flavum [29]. The initial studies of indirect decompression were standalone procedures without posterior fixation and hence more incidence of cage subsidence and subsequent loss of correction [16]. Fujibayashi et al. [9] showed that posterior percutaneous fixation not only increases the stability of the construct but also prevents cage subsidence and consequent loss of correction. Lin et al. [30] have shown that clinical results of OLIF are equivalent to MI-TLIF whereas radiographic results of OLIF are superior that MI-TLIF. Wang et al. [31] has shown that only bony lateral canal stenosis is a contraindication for indirect decompression and states that significant canal stenosis can still undergo indirect decompression with expectation of good clinical outcome and radiographic improvement. Our study seconds this as only one case of direct posterior decompression was required and it was also due to cage subsidence and consequent loss of decompression.
1. Limitations
The limitations of this study include a small sample size and short radiographic follow-up. Maintenance of correction and decompression was not evaluated in long term. It is important to note what happens in the long term if subsidence occurs and whether in long term there is restenosis and if delayed posterior decompression is required. The second limitation is the retrospective nature of the study. This will have a selection bias for patients in which indirect decompression was offered in severe canal stenosis. Further Prospective randomized studies are required to exactly answer the research question of the efficacy of indirect decompression in severe lumbar canal stenosis. It would be also important to have a comparative control study with posterior direct decompression and indirect decompression to find out the difference. Lastly, fusion rates for the OLIF approach will need to be assessed as this is one of the goals of surgery for long-term success.
CONCLUSION
Indirect decompression is effective in severe lumbar central canal stenosis in Schizas grade C and D. OLIF is an effective means of indirect decompression with early excellent to good results clinically. There is improvement in disc height, foraminal height, segmental lordosis, and overall spinal canal area. Posterior direct decompression is unnecessary in the majority of cases and spares the patient undue morbidity and risk of neural injury or scarring from direct posterior surgery. Further research is needed to identify predictive factors in patients with severe central spinal canal stenosis who may benefit from OLIF.