AbstractObjectiveThis study aimed to evaluate the radiologic and clinical outcomes of minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) and conventional posterior lumbar interbody fusion (PLIF) at the L5–S1.
MethodsWe retrospectively reviewed patients who underwent posterior lumbar fusion (MIS-TLIF and PLIF) at only the L5–S1 and were followed up for more than 12 months. Age, sex, body mass index (BMI), bone mineral density (BMD), diagnosis, comorbid conditions, fusion rate, perioperative results, and pre- and postoperative radiographic parameters at the L5–S1 level, pelvic parameters and degree of spondylolisthesis, and clinical results were analyzed.
ResultsA total of 102 patients (46 male, 56 female) with a mean age of 57.1 years were evaluated. Fifty and fifty-two patients underwent MIS-TLIF and PLIF surgeries, respectively. Radiologic parameters increased from their preoperative measures at the last follow-up study; similarly, there were no intergroup differences. The fusion rates in the MIS-TLIF and PLIF groups were 86% and 82.7%, respectively. The subsidence rates in the MIS-TLIF and PLIF groups were 6% and 3.8%, respectively. There was no intergroup difference in terms of fusion rate and subsidence. Clinical outcomes also gradually improved after surgery in both groups without intergroup differences.
INTRODUCTIONTransforaminal lumbar interbody fusion (TLIF) was introduced by Harms and Rolinger [1] in 1982 and has been still widely used until recently. TLIF usually approaches the disc space through one-sided facetectomy, which has the advantage of reducing tissue damage and thecal sac retraction. Minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) uses a tubular retractor and percutaneous pedicle screw fixation system to reduce the requirement of skin incisions, blood loss, and postoperative pain, thereby reducing the duration of hospital stay [2-4].
MIS-TLIF has many such advantages for patients, but some surgeons doubt its usefulness at the L5–S1. Structures containing the L5–S1 have been of particular interest because of their unique anatomy, the transition from the movable segment to the stationary segment, which results in higher mechanical stress and loads compared to other segments of the lumbar spine [5,6]. For this reason, several studies have revealed that the fusion rate of L5–S1 is lower than that of other lumbar segments [7-9]. Although many studies have examined the clinical and functional outcomes of conventional posterior lumbar interbody fusion (PLIF) surgery between L5–S1 level and other lumbar levels, none have compared radiologic and clinical outcomes and fusion rates between MIS-TLIF and conventional PLIF limited to the L5–S1.
This study aimed to evaluate the radiologic and clinical outcomes of MIS-TLIF and conventional PLIF at the L5–S1. This research question was based on how the unique structure of L5–S1 affects the union of MIS-TLIF and conventional PLIF. Therefore, it is necessary to examine patients’ radiologic parameters and functional outcomes. We hypothesize that fusion rates after MIS-TLIF and PLIF were equivalent in L5-S1 level.
MATERIALS AND METHODS1. PatientsThis retrospective study was performed at a single institution. A total of 580 consecutive patients who underwent posterior lumbar fusion (69 MIS-TLIF, 511 conventional PLIF) at only the L5–S1 level were enrolled. The inclusion criteria were as follows: 1) age>18 years; 2) symptomatic spinal stenosis, spondylolisthesis, or herniated lumbar disc disease; 3) at least 12 months of follow-up; and 4) underwent postoperative computed tomography (CT) to confirm fusion. Patients with tumors, infection, trauma, or multilevel fusion were excluded. Two-hundred-and-eighty-five patients were excluded from our study because they underwent multi-level surgery or were lost to follow-up. The first cohort of 50 patients with MIS-TLIF was matched with 52 patients from the group with conventional PLIF surgery. All patients were matched with a patient of the sex, age and diagnosis. Then we had two matched cohorts (I and II) of 50 and 52 patients, respectively (Figure 1).
These patients underwent mono-segmental posterior lumbar interbody fusion at L5–S1 using MIS-TLIF (Figure 2A) or conventional PLIF (Figure 2B) with a screw and double rod system and a polyetheretherketone (PEEK) cage with a 10° lordotic angle according to the different surgical methods performed by one different senior surgeon with more than 10 years’ surgical experience. All patients underwent CT and radiography before surgery and at least 1 year after surgery. This study was reviewed and approved by our local institutional review board (IRB No: 3-2021-0378).
2. Surgical ProcedureMIS-TLIF is performed through a standard 2–3 cm paramedian approach, with the patient in the prone position. Using a tubular retractor (METRx®; Medtronic Sofamor Danek, Memphis, TN, USA), the incisions were opened one after the other and a 24-mm working channel was inserted. A facetectomy was performed on the unilateral side. The ascending and descending articular processes were removed using an osteotome and drill. The foraminotomy was performed unilaterally or bilaterally according to the patient’s symptoms. Complete discectomy and end plate preparation were performed using pituitary rongeurs and curettes. After discectomy, autologous bone from the laminectomy filled the disc space. A single lordotic PEEK cage (Capstone; Medtronic Sofamor Danek) which was filled with only autologous local bone fragment were inserted into the disc space. Then, a percutaneous pedicle screw and rod system (Sextant; Medtronic Sofamor Danek) were inserted under C-arm fluoroscopic images [10].
Conventional PLIF was performed through a midline approach in the prone position. Laminectomy and bilateral medial facetectomy were performed using a Carrison rongeur and drill. Complete discectomy and endplate preparation were performed as in MIS-TLIF. Two PEEK cages (Concorde; Dupey Synthes, West Chester, PA, USA) was filled with autologous bone and inserted into the disc space. Subsequently, bilateral pedicle screw and rod fixation (CD Horizon Legacy; Medtronic Sofamor Danek) was performed [11].
3. Radiographic AssessmentRadiological evaluation was performed using plain radiography (standing lumbosacral anteroposterior, lateral, flexion, extension, whole spine anteroposterior, and lateral) and CT scans preoperatively, immediately postoperatively, and at 12 months postoperatively. Disc height (DH), disc slope angle (DSA), disc angle (DA), segmental lordotic angle (SLA) at the L5–S1 level, pelvic parameters, and degree of spondylolisthesis were measured using X-ray scanning.
The DH measured the distance between the L5 lower endplate and the S1 upper endplate, which is measured as the sum of a line drawn in the middle between the endplates and a vertical line drawn from the corners of the upper and lower segmental vertebrae, then divided in half (Figure 3) [12]. The DA was the angle between the L5 lower endplate and the S1 upper endplate. DSA was defined as the angle between the horizontal line and the line connecting the midpoint between the anterior and posterior disc spaces [7]. The SLA was measured in degrees between the L5 upper endplate and the S1 upper endplate (Figure 4). Pelvic parameters included the lumbar lordotic angle (LL), pelvic incidence (PI), pelvic tilt, PT, and sacral slope (SS). The degree of spondylolisthesis was measured as the percentage of the distance between the posterior border of the L5 vertebra and the posterior border of the rostral vertebra.
Bony fusion, cage subsidence, and hardware failure were assessed on CT. Lumbar spine CT scan was performed preoperatively and at 12 months postoperatively. The modified Bridwell fusion criteria [13,14] were also measured on CT scans. Using these criteria, the fusion rate could be defined as follows: grade 1, fused with remodeling and trabeculae present; grade 2, graft intact, not fully remodeled and incorporated, but no lucency present; grade 3, graft intact, potential lucency present at the graft’s top and bottom; and grade 4, fusion absent, with graft collapse/resorption. Therefore, fusion was identified when grade 1 or 2 was accepted through these criteria. Subsidence means that the disc height was lowered by 2 mm or more in the follow-up plain lateral X-ray scan.
4. Functional EvaluationDemographic and baseline data included the number of patients, sex, age at surgery, postoperative outpatient follow-up duration, body mass index (BMI), bone mineral density (BMD), primary diagnosis, and comorbid conditions such as diabetes mellitus and smoking status were recorded. Operation time, estimated blood loss, and length of hospital stay were also assessed from the patients’ hospital records. We used the visual analog scale (VAS) and the Oswestry Disability Index (ODI) to compare the MIS-TLIF and PLIF groups preoperatively, immediately postoperatively, and at 1 year postoperatively.
5. Statistical AnalysisThe statistical analysis was performed using SPSS version 25.0 software (IBM Corporation, Armonk, NY, USA). All continuous data are presented as mean and standard deviation and were tested for normal distribution using the Kolmogorov–Smirnov test. Differences in baseline data and radiologic parameters were analyzed using the t-test or the Mann–Whitney U test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Statistical significance was set at p<0.05.
RESULTS1. Patients DemographicsA total of 102 patients (50 in the MIS-TLIF group, 52 in the PLIF group) were included in the study. The patients’ demographic characteristics are shown in Table 1. The mean age at surgery (57.00 vs. 57.17 years; p=0.929) and mean follow-up duration (23.42 vs. 22.37 months; p=0.791) did not differ significantly between groups. The BMI (25.02 vs. 25.06 kg/m2; p=0.945) and BMD (–1.55 vs. –1.41; p=0.591) were also comparable. In both group, isthmic spondylolisthesis was the main pathology (20/50 [44%] and 22/50 [42.3%]).
2. Radiologic OutcomesAll radiologic parameters are presented in Table 2. Radiologic parameters were evaluated using the preoperative, immediately postoperative, and 12 months postoperative plain radiographs as mentioned above. The pre- and postoperative DH did not differ significantly between the two groups immediately postoperatively or at 12 months postoperatively. DA and DSA did also not differ significantly between the two groups in the preoperative and postoperative states. However, the SLA of L5–S1 was 16.5° and 18.34° in the MIS-TLIF group and 13.95° and 17.10° in the PLIF group pre- and postoperatively, respectively. There was a significantly difference between the preoperative SLA of L5–S1 (p=0.047).
LL and SS increased slightly after surgery in both groups, but the difference was not significant. There was no significant difference in pre- or postoperative PI in either group. PT decreased slightly after surgery; however, there was no significant difference in either group. The pre- and postoperative values of the spondylolisthesis rate in patients with both degenerative and isthmic spondylolisthesis were reduced, and there was no significant intergroup difference.
Table 3 shows the perioperative results and fusion rates of the two groups. Regarding perioperative outcomes, the operation time and the estimated blood loss were significantly lower in the MIS-TLIF group (MIS-TLIF: 101.92 min, 164.8 mL vs. PLIF: 172.27 min, 675.38 mL; p<0.001). There was no significant intergroup difference in the length of hospital stay (MIS-TLIF, 11.14 vs. PLIF, 12.63 days; p=0.285). The fusion rate was analyzed using the modified Bridwell fusion criteria. The fusion rate of the MIS-TLIF group was 86%, while that of the PLIF group was 82.7%. Cage subsidence was observed in 6% (3/50) of the patients with MIS-TLIF and 3.8% (2/52) of PLIF patients. However, there were no statistically significant intergroup differences.
3. Functional OutcomesThe mean VAS scores notably decreased in both groups from 5.94±2.24 preoperative to 2.50±1.49 immediately postoperatively and 1.52±1.73 at 12 months postoperatively in the MIS-TLIF group, and from 5.81±2.11 preoperatively to 2.15±1.90 immediately postoperatively and 1.92±1.88 at 12 months postoperatively in the PLIF group, respectively. The perioperative mean VAS score in both groups improved significantly, and there was no intergroup difference. Likewise, there were significant improvements in the ODI in both groups: from 41.41±13.51 preoperatively to 24.68±9.29 immediately postoperatively and 16.32±8.90 at 12 months postoperatively in the MIS-TLIF group, and from 48.27±13.31 preoperatively to 27.87±9.37 immediately postoperatively to 20.13±7.15 at 12 months postoperatively in the PLIF group. There were no significant intergroup differences.
DISCUSSIONMinimally invasive spinal fusion surgery has been widely used for the treatment of lumbar degenerative diseases, with many advances. MIS-TLIF uses a tubular retractor localized in the paraspinal area to prevent excessive muscle destruction. Additionally, unlike PLIF, MIS-TLIF does not require dural or nerve root over retraction, reduces dural damage, and can reduce intraoperative bleeding with smaller incisions than in conventional PLIF [3,10,15]. As it is possible to increase the foraminal height through removal of the facet complex, foraminal stenosis is also a suitable indication for MIS-TLIF. In addition, as reported in many other studies, MIS-TLIF is preferred for degenerative and isthmic spondylolisthesis [16-18]. The L5–S1 is one of the most affected levels of spondylolisthesis, especially in patients with isthmic spondylolisthesis. In our study, the fusion rate of L5–S1 was 86% in the MIS-TLIF group and 82.7% in the PLIF group, which was similar to the results of previous studies but lower than that of other lumbar levels. Ito et al. [19] reported a fusion rate of 96.4% at L4–L5 and 87.5% at L5–S1. Han et al. [7] reported a fusion rate of 89.8% (53/59) at L4–L5 and 42.9% (6/14) at L5–S1. Bassani et al. [20] compared posterior and anterior techniques in single-level L5–S1 interbody fusion and reported that the fusion rate of ALIF was 88.65%, while that of TLIF was 91.9% (p=0.23). Mun et al. [21] reported a fusion rate of both oblique lumbar interbody fusion (OLIF) and TLIF at L5–S1 single level. Accordingly, the fusion rates were 81.8% and 87.8% for OLIF and TLIF, respectively. This low fusion rate for L5–S1 may be due to several factors.
First, the DSA was higher than at other lumbar levels. In our study, the DSA of L5–S1 was 28.40°, twice as high as that of L4–L5 in a previous study [7]. As the DSA increased, the shear force in the disc space also increased. Therefore, this may be a factor of the fusion rate being lower for L5–S1 than for L4–L5. Second, the compression strength of L5–S1 is lower than that of L4–L5 [22]. Because the lordosis of the lumbar spine originates from the L3 or L4 vertebrae, the compressive strength may be lower than that of the L5 vertebra. Third, the range of motion of L5–S1 was greater than that of other lumbar vertebral levels. This means that after PLIF, translational motion between the L5 vertebra and the sacrum may occur on a larger scale than at other lumbar segments. Fourth, the conical shape of the L5–S1 disc space also lowered the fusion rate. The posterior margin of L5–S1 is narrower than the anterior margin compared to that in other lumbar levels [7,23]. Because the entrance is narrow, if the cage is placed in the same way as in other lumbar levels, a large space is left, which lowers the fusion rate. Because of the low fusion rate of L5–S1, this study attempted to increase the contact area of the cage and increase the contact force between the cage and the bony endplate. In addition, to reduce the empty space in the disc space after cage insertion, one must maximize bone packing in the disc space to improve the fusion rate. In addition, a larger cage was inserted by drilling or osteotomy of part of the caudal endplate of L5 and part of the cranial endplate of S1. For these reasons, the fusion rates of MIS-TLIF and conventional PLIF surgery were improved in this study compared to those in previous studies.
1n a previous study, TLIF could cause a kyphotic change; in another study, 57% of patients who were normal before surgery developed lumbar kyphosis after TLIF [24,25]. Kyphosis progression was also observed in MIS-TLIF [26,27]. However, in several studies, LL and SLA improved after MIS-TLIF. Le et al. [28] reported that LL was improved or preserved in 86% of cases, while SLA improved in 97.4% of cases. Choi et al. [29] reported that LL improved from 44.3° to 47.1° at 12 months postoperative, while SL improved from 13.3° to 15.5° at 12 months after surgery. In our study, LL improved from 33.57° to 35.59° at the last follow-up, while SL improved from 16.5° to 18.34° at the last follow-up. This showed the same results in MIS-TLIF and conventional PLIF surgery, with no significant intergroup differences. This is because the cage was placed as high as possible anterior to the disc space and the angle was made through sufficient compression of the rod between screws.
In most cases, the reduction of spondylolisthesis is performed using open techniques as the general standard of care. However, surgical management of spondylolisthesis using MIS-TLIF has been studied extensively, and the clinical results are good [30,31]. In recent studies, techniques for reduction of spondylolisthesis such as the “swing technique” and “rocking technique” have been introduced, and correction was successfully performed through improvement of segmental deformity and reduction in spondylolisthesis patients [32,33]. Kim et al. [30] reported that the spondylolisthesis rate was reduced from 16.77% to 9.79% with MIS-TLIF in isthmic spondylolisthesis and from 11.33% to 3.78% in degenerative spondylolisthesis. In our study, the spondylolisthesis rate was reduced from 20.17% to 10.01% in the MIS-TLIF group and from 22.44% to 10.51% in the PLIF group. There was no statistically significant difference in reduction between the two surgeries, and spondylolisthesis was corrected.
In our study, VAS and ODI were significantly improved after both MIS-TLIF and conventional PLIF surgery. In addition, there was no significant intergroup difference in the improved results. This means that the results are equivalent between the MIS-TLIF and conventional PLIF procedures. However, Cheng et al. [34] showed that although there was no difference in VAS after surgery, the use of morphine or equivalent pain medication during hospitalization was lower after MIS-TLIF. This is because MIS-TLIF features a smaller skin incision and less intraoperative bleeding. In our study, compared to conventional PLIF, the operative time and the amount of perioperative bleeding were significantly less for MIS-TLIF. This means that the burden on patients and surgeons was reduced.
There were some limitations to this study. First, it was a retrospective study conducted at a single center. Second, the follow-up period was comparatively short. However, this is the first comparative study of fusion rate through the analysis of radiological parameters of patients who underwent MIS-TLIF and conventional PLIF at the L5–S1 level. Further studies involving long-term follow-up with a multicenter study should be performed.
CONCLUSIONBoth MIS-TLIF and conventional PLIF at the L5–S1 level showed good clinical and radiological outcomes. Although there was no significant intergroup difference in postoperative VAS and ODI values, MIS-TLIF was more effective than conventional PLIF because it reduced burden on the patient and surgeon due to the small amount of perioperative bleeding and short operation time.
Figure 1.Flowchart of the patient selection process. MIS-TLIF: minimally invasive transforaminal interbody fusion, PLIF: posterior lumbar interbody fusion. 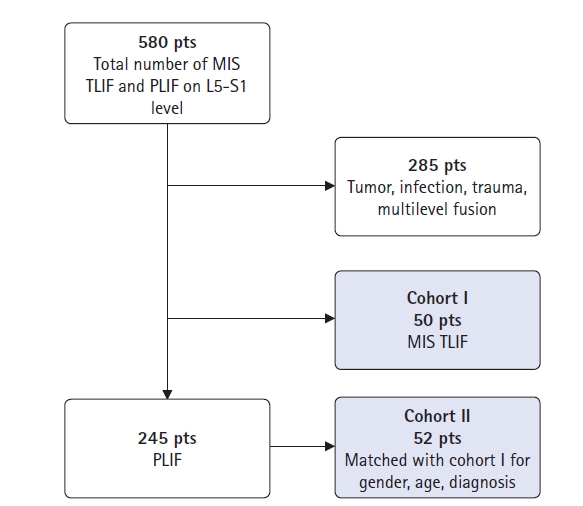
Figure 2.Lumbar spine lateral radiographs (A) showing the posterior lumbar interbody fusion at L5–S1 using MIS-TLIF and (B) showing the conventional PLIF. 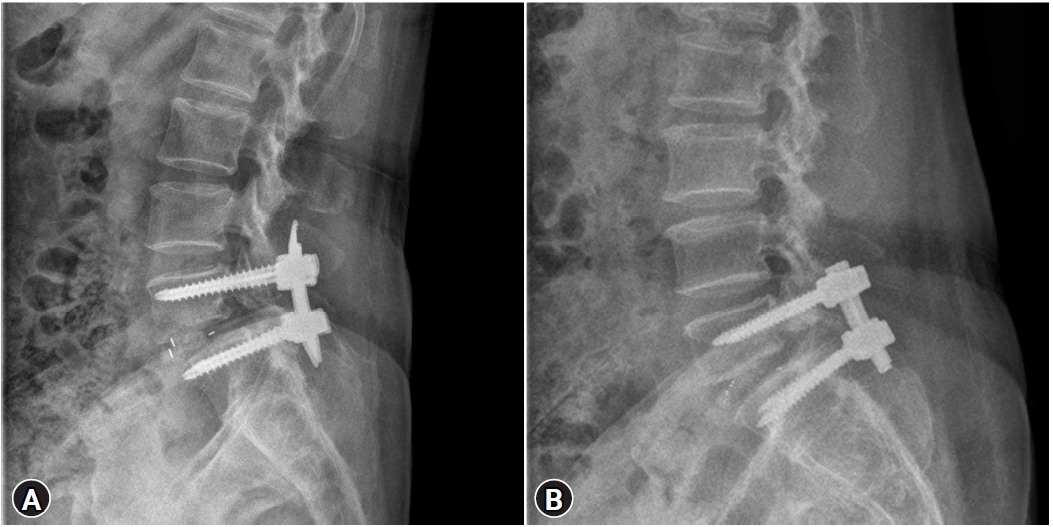
Figure 3.Disc height measurement according to the Frobin method. The four corners of the vertebral segments are designated on the lateral radiographs (1, 2, 3, 4). The medial point, medial plane (median line), and its bisector are shown. Disc height is the sum of the lines perpendicular to the bisector from the corners of the upper and lower segmental vertebrae divided in half. Disc height=(H1+H2+h1+h2)/2. 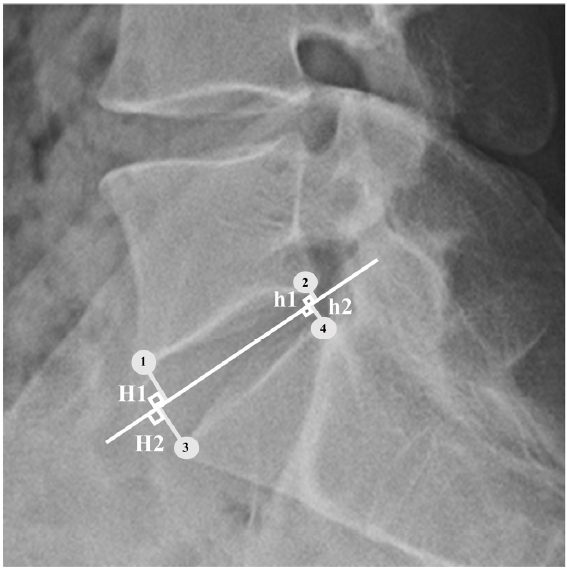
Figure 4.Overview of the radiographic parameters. Disc angle (DA): angle between the L5 lower endplate and the S1 upper endplate (black line). Disc slope angle (DSA): angle between the horizontal line and the line connecting the midpoint between the anterior and posterior of disc spaces (gray line). Segmental lordotic angle (SLA): angle between the L5 upper endplate and the S1 upper endplate (white line). 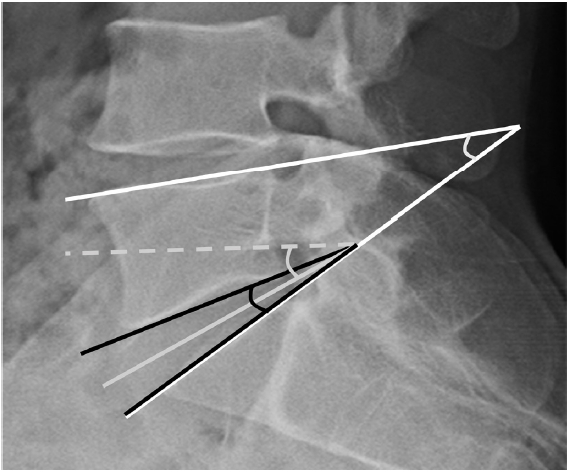
Table 1.Patients’ demographic and baseline data
Table 2.Patients’ radiological parameters by group
Table 3.Patients’ perioperative results and fusion rates by group
REFERENCES1. Harms J, Rolinger H. Die operative Behandlung der Spondylolisthese durch dorsale Aufrichtung und ventrale Verblockung [A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author’s transl)]. Z Orthop Ihre Grenzgeb 1982 120:343–347. German.
2. Gum JL, Reddy D, Glassman S. Transforaminal lumbar interbody fusion (TLIF). JBJS Essent Surg Tech 2016;6:e22.
3. Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J 2012;21:2265–2270.
4. Parker SL, Mendenhall SK, Shau DN, Zuckerman SL, Godil SS, Cheng JS, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg 2014;82:230–238.
5. Tsuchiya K, Bridwell KH, Kuklo TR, Lenke LG, Baldus C. Minimum 5-year analysis of L5-S1 fusion using sacropelvic fixation (bilateral S1 and iliac screws) for spinal deformity. Spine (Phila Pa 1976) 2006;31:303–308.
6. Pateder DB, Park YS, Kebaish KM, Cascio BM, Buchowski JM, Song EW, et al. Spinal fusion after revision surgery for pseudarthrosis in adult scoliosis. Spine (Phila Pa 1976) 2006;31:E314–E319.
7. Han SH, Hyun SJ, Jahng TA, Kim KJ. A comparative radiographic analysis of fusion rate between L4-5 and L5-S1 in a single level posterior lumbar interbody fusion. Korean J Spine 2015;12:60–67.
8. Agazzi S, Reverdin A, May D. Posterior lumbar interbody fusion with cages: an independent review of 71 cases. J Neurosurg 1999;91:186–192.
9. Ito Z, Matsuyama Y, Sakai Y, Imagama S, Wakao N, Ando K, et al. Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion. Spine (Phila Pa 1976) 2010;35:E1101–E1105.
10. Lee CK, Park JY, Zhang HY. Minimally invasive transforaminal lumbar interbody fusion using a single interbody cage and a tubular retraction system : technical tips, and perioperative, radiologic and clinical outcomes. J Korean Neurosurg Soc 2010;48:219–224.
11. DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. J Am Acad Orthop Surg 2008;16:130–139.
12. Frobin W, Brinckmann P, Biggemann M, Tillotson M, Burton K. Precision measurement of disc height, vertebral height and sagittal plane displacement from lateral radiographic views of the lumbar spine. Clin Biomech (Bristol, Avon) 1997;12 Suppl 1:S1–S63.
13. Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic, lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects. Spine (Phila Pa 1976) 1995;20:1410–1418.
14. Bridwell KH, O’Brien MF, Lenke LG, Baldus C, Blanke K. Posterior spinal fusion supplemented with only allograft bone in paralytic scoliosis. Does it work. Spine (Phila Pa 1976) 1994;19:2658–2666.
15. Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976) 2000;25:1437–1446.
16. Park Y, Ha JW, Lee YT, Sung NY. Minimally invasive transforaminal lumbar interbody fusion for spondylolisthesis and degenerative spondylosis: 5-year results. Clin Orthop Relat Res 2014;472:1813–1823.
17. Pan J, Li L, Qian L, Zhou W, Tan J, Zou L, et al. Spontaneous slip reduction of low-grade isthmic spondylolisthesis following circumferential release via bilateral minimally invasive transforaminal lumbar interbody fusion: technical note and short-term outcome. Spine (Phila Pa 1976) 2011;36:283–289.
18. Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J 2010;19:1780–1784.
19. Ito Z, Imagama S, Kanemura T, Hachiya Y, Miura Y, Kamiya M, et al. Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion (PLIF): a multicenter study. Eur Spine J 2013;22:1158–1163.
20. Bassani R, Morselli C, Querenghi AM, Nuara A, Sconfienza LM, Peretti GM. Functional and radiological outcome of anterior retroperitoneal versus posterior transforaminal interbody fusion in the management of single-level lumbar degenerative disease. Neurosurg Focus 2020;49:E2.
21. Mun HY, Ko MJ, Kim YB, Park SW. Usefulness of oblique lateral interbody fusion at L5-S1 level compared to transforaminal lumbar interbody fusion. J Korean Neurosurg Soc 2020;63:723–729.
23. Kimura H, Shikata J, Odate S, Soeda T, Yamamura S. Risk factors for cage retropulsion after posterior lumbar interbody fusion: analysis of 1070 cases. Spine (Phila Pa 1976) 2012;37:1164–1169.
24. Madhavan K, Shamrock AG, Chieng LO, Vanni S, Wang MY. Assessment of sagittal balance post TLIF - are we kyphosing the lumbar spine? Paper presented at: 2017 AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves; 2017 Mar 8-11; Las Vegas, NV.
25. Dorward IG, Lenke LG, Bridwell KH, OʼLeary PT, Stoker GE, Pahys JM, et al. Transforaminal versus anterior lumbar interbody fusion in long deformity constructs: a matched cohort analysis. Spine (Phila Pa 1976) 2013;38:E755–E762.
26. Lee HJ, Kim JS, Ryu KS. Minimally invasive TLIF using unilateral approach and single cage at single level in patients over 65. Biomed Res Int 2016;2016:4679865.
27. Shen X, Wang L, Zhang H, Gu X, Gu G, He S. Radiographic analysis of one-level minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) with unilateral pedicle screw fixation for lumbar degenerative diseases. Clin Spine Surg 2016;29:E1–E8.
28. Le H, Anderson R, Phan E, Wick J, Barber J, Roberto R, et al. Clinical and radiographic comparison between open versus minimally invasive transforaminal lumbar interbody fusion with bilateral facetectomies. Global Spine J 2021;11:903–910.
29. Choi WS, Kim JS, Ryu KS, Hur JW, Seong JH. Minimally Invasive transforaminal lumbar interbody fusion at L5-S1 through a unilateral approach: technical feasibility and outcomes. Biomed Res Int 2016;2016:2518394.
30. Kim JY, Park JY, Kim KH, Kuh SU, Chin DK, Kim KS, et al. Minimally invasive transforaminal lumbar interbody fusion for spondylolisthesis: comparison between isthmic and degenerative spondylolisthesis. World Neurosurg 2015;84:1284–1293.
31. Massel DH, Mayo BC, Shifflett GD, Bohl DD, Louie PK, Basques BA, et al. Minimally invasive transforaminal lumbar interbody fusion: comparison of isthmic versus degenerative spondylolisthesis. Int J Spine Surg 2020;14:115–124.
32. Park B, Noh SH, Park JY. Reduction and monosegmental fusion for lumbar spondylolisthesis with a long tab percutaneous pedicle screw system: “swing” technique. Neurosurg Focus 2019;46:E11.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||