AbstractObjectiveExecutions of indications/extended indications are associated with higher than normal rates of symptomatic recurrences and treatment failures, especially for novice surgeons incorporating Percutaneous Transforaminal endoscopic lumbar discectomy/decompression (PTELD) techniques. Causes of failures can be manifold and can occur because of a residual or a complete fragment causing persistent compression or associated unaddressed stenosis. To prevent this problem, proper training, multiple instrument inventory, variable techniques are needed with progressive learning. Authors aim to suggest objective and subjective criteria to define end-points/adequacy of decompression (EPD).
MethodsPubMed database search was limited to locate only adequacy of decompression of PTELD and thus included specific keywords: “ENDPOINT” OR “ADEQUATE” AND “DECOMPRESSION” AND “TRANSFORAMINAL” AND “ENDOSCOPY”. Authors added their experience to refine and define multiple EPD.
ResultsIn the search we found 12 articles total. Upon reviewing these, we found 7 articles matching our criteria. Cross references of included articles were searched, 5 additional articles were included. EPD were described in only 9 articles. Author’s experience with other relevant references were added to complete the viewpoint (EPD, n=29). Direct observed/provoked EPD and inferred EPD were defined separately. Videos, illustrations and descriptions of each EPD are illustrated to provide the ideation.
INTRODUCTIONIn the last quarter of 20th century and early 21st century, PTELD has rapidly evolved as an alternative for lumbar disc herniations (LDH) [1]. Advantages of PTELD are remarkable due to surgery under local anaesthesia (LA), though general (GA) and regional anaesthesia can also be used. Minimal damage to muscles/bone/other soft vertebral tissue restraints, rapid recovery, nominal post-operative pain, reduced procedure related morbidity, and a high patient satisfaction rate [2,3] are the other specific advantages. The technique of PTELD has come a long way in the last few decades through experiences of pioneers along with numerous advancements in the field of optics, instrumentation and enabling technology (Magnetic Resonance Imaging [MRI], Radio-frequency [RF], Laser, Ultrasound, Navigation etc.) [4,5]. Initial cases of discectomy were limited to soft para-central LDH. But, gradually it has also been used for treating central, highly migrated, foraminal and extraforaminal LDH, cauda equina syndrome (CES), lumbar spinal stenosis (LSS), stable listhesis, separation surgery, discitis/epidural abscesses and also for performing fusion surgeries [1,6-12].
However, these extended indications and increasing surgical numbers may be associated with higher rates of symptomatic recurrences and treatment failures, especially for novice surgeons incorporating newer ESS techniques [13]. The endpoint of PTELD needs to be identified and differentiated; it is different when PTELD is used only as a pain procedure and when decompression is the surgical goal. Pre-operative evaluation and case selection is of utmost importance in any ideal or advanced indication of ESS. The authors in this review article aim to describe objective and subjective criteria to define the end-point/adequacy of decompression (EPD) in PTELD.
MATERIALS AND METHODSFor this narrative review, database search was limited to locate only adequacy of decompression of a transforaminal endoscopic lumbar surgery. The search words were limited to specific keywords: “ENDPOINT” OR “ADEQUATE” AND “DECOMPRESSION” AND “TRANSFORAMINAL” AND “ENDOSCOPY”. We started the search with above mentioned keywords and the search was done in “PubMed” data base [14]. Required articles language was English. Additional repeat confirmation by two independent researcher reviewers were done for validation and confirmation of the literature review. None of the correspondent authors were contacted for any kind of doubt or other query & resolution. Author’s experience with the other needed references are added to further refine and define EPD. Key literature pertinent to the current topic have been cited and emphasis has been placed on literature published within the last decade to provide the most current recommendations.
RESULTSIn the search, we found a total 12 articles (Figure 1). Upon reviewing the 12 articles, we found 7 articles matching with our criteria and the 5 articles that were excluded were unrelated to ESS. Cross references of the included articles (7) were searched and 5 additional articles were included. EPD were defined in 9 articles only and they were tabulated (Table 1). In PTELD the suggested EPD is by pre-operative planning, achieving the planned EPD with its optimal confirmations per-operatively and documenting decompression post-operatively by objective and subjective parameters. Authors re-identified and describe 29 EPD’s separately. Videos, illustrations and descriptions of each of the EPD is added to prove the ideation.
DISCUSSIONIn MIS (minimally invasive spine surgery) or more importantly in ESS (endoscopic spine surgery), the optimum decompression goal should get as close to the COSS (conventional open spine surgery) decompression goal. This needs very specific diagnosis and target unlike COSS which focuses on an exploratory decompression solutions and hence is more forgiving [15]. It is known that for Inter-laminar ESS (Unilateral Biportal, Destandeau, Uniportal Interlaminar Lumbar Decompression) [16-19] the endpoint is still the same as COSS. However, in PTELD the suggested EPD is three tier. Firstly by pre-operative planning, then achieving the planned EPD with its optimal confirmations per-operatively and finally documenting decompression post-operatively by objective and subjective parameters. Factors influencing the EPD include the underlying pathology (LSS vs. LDH: soft/hard LDH), level of surgery, the site of compression (dorsal/ventral), approach technique used (IO: Inside Out/OI: Outside In, and FEE: Flat Entry Epidural [1]), duration of symptoms (acute/chronic), radiological patient specific features, experience, school of thought and expertise of the surgeon.
1. Pre-operative Planning for Target EPDWe recommend an immediate pre-operative MRI (Magnetic Resonance Imaging) to assess the pathology and for clinico-radiological correlation. This is not only for pre-operative planning, but also to assess the size of fragment for intra-operative assessment of completion of surgical decompression. Not to forget that there may be a change in the location of the fragment after initial presentation, which can only be confirmed by an immediate pre-operative screening MRI. This is essential to curtail chances of a missed fragment, as PTELD is a minimalistic procedure and does not allow change of trajectory of approach and visualization as compared to exploratory COSS [20]. Plotting of LDH fragment and stenosis can be drawn on the radiographs (antero-posterior and lateral views) (Figure 2D, E). This is helpful during initial learning because stenosis or spatial configuration of LDH fragments in spinal canal is based on 2D MRI films or 3D imagined PACS visualization. But, our per-operative endoscope tip and working instruments jaws location is guided on image intensifier radiographic images only.
2. Intra-operative Assessment of EPD1) EPD in PTELD for LDHThe transforaminal approach in “inside out” technique refers to a postero-lateral approach to the disc or epidural space through the foraminal window [21]. The detailed techniques can be referred to in previous literature and is not the focus of present study [1, 22-24]. EPD defined in previously published literature in the PubMed search is limited (Table 1). The Author’s recommend multiple EPD in addition to previously mentioned literature EPD (Table 2). For ease of understanding many non provocative and provocative direct EPD along with inferred EPD are suggested. After multiple release and decompression techniques, confirm decompression by direct and indirect evidences. Though debatable, we also believe that the direct visualization of neural elements is the best documentation to avoid failures [25]. Authors’ EPD includes complete direct visualization of the Traversing Nerve Root (TNR) when it is the focus of decompression. The extent of direct visualization is from superjacent lower endplate unto the inferjacent pedicle by angulating the endoscope. This can be further extended in cases of up-migrated or down-migrated fragments by doing additional maneuvers like a partial pediculectomy. In cases with central LDH, central stenosis or CES, direct visualization of the central dural sac is taken as the EPD [1]. Once the EPD is reached, neural fall back occurs and neural tissue occupies the space that was previously occupied by LDH fragment (Figure 3).
These EPD vary depending on the pathology. In para-central protrusions even an intact annulus with free epidural fat pop-out signals adequate decompression [1]. This fat pop-out is usually seen in acute LDH, para-central LDH and is more pronounced in high BMI (Body Mass Index) patients (Figure 4). In fact, the fat may hinder in actual visualization of decompressed neural structures. In these type of cases, probing along the length of TNR is especially important to avoid false positive EPD. In cases of LDH extrusion, major fragment retrieval (Figure 5, Supplementary Video 1), i.e., removal of the culprit fragment is the best sign of a complete decompression and EPD. Fragmented epidural components may remain and should be looked for especially when migrated or extruded fragments with more than a week-old presentation are addressed. This is more likely when epidural trans-PLL (posterior longitudinal ligament) fragment is present due to the body’s phagocytic response to the antigenic nucleus pulposus [26,27]. This major fragment retrieval does not occur in disc bulge, stenosis and calcification. In this later type of cases slow crab eating technique is what is needed. Usually, a gush of epidural blood comes out, called as “Red-Out” after the fragment retrieval indicating unobstructed epidural space (Figure 6) [1,28]. Visibility is always gained in a while with fluid ingress and washout, when the dura expands and creates a normal haemostatic tamponade. False positive red out are also noted in sudden multiple bleeder points especially with cutting burr, major bleed or any fluid inflow blockade in the endoscope [28]. After the fragment removal is achieved, opposite annular fissure visualization is possible (Figure 3, 7). This is the EPD in a protrusion or extrusion followed by most experienced surgeons (Supplementary Video 2). Habit of eyeballing the size of fragment (Figure 8) removed by the instrument and matching it with an extruded or sequestrated fragment size on MRI should be the dictum. If in any case with fragmented nucleus (extrusion, sequestration) there is no red coloured part in the removed disc pieces, then it suggests that the culprit nuclear fragment is still pending to be removed. The red area depicts the epidural outer surface (Figure 5), while yellowish area is unexposed part, though degenerated. Sometimes, a whitish coloured area is noted and is the most inside part of the major fragment within the disc space confines itself (Figure 9). At the same time a red staining fragment retrieved but not matched to size on MRI should arouse suspicions. Small fragment miss outs are common in sequestration. Use of live dye Indigo-carmine is used for spotting the fragment easily and identifying degenerated fragments. This is useful for EPD judgment and used at many centres worldwide [29,30].
In extra-foraminal or foraminal LDH, the exiting nerve root is visualized and probed below it against the vertebral body and the disc annulus after retrieving the fragments. Many times crab eating of the compressing annular hardening, calcified LDH and ventral LSS is required. Variable use of burr, osteotomes, hooks, dissectors and articulated scoops are needed to decompress them optimally. Once the EPD is reached in these cases there is neural fall back and visualization of the TNR and dural sac. In cases of sub annular LDH, the PLL will be free and is considered as the EPD. Depending on the location of the annular tear, annular flap mobility must be considered as the EPD (Figure 7, Supplementary Video 2). In cases where there is no annular flap mobility, look for chronicity features: end plate spur/hardened annulus (Figure 10). This may help locate the associated stenosis in addition to a disc fragment. If it is tackled optimally, it will avoid a failure. These are all “non-provocative EPD”.
When the EPD is reached, movements of the dural sac and neural elements can be elicited by various “Provocative” maneuvers. Visualization of strong epidural pulsation of the and TNR are the confirmed EPD. EP can be seen in-sync with the patient’s breathing as well (Supplementary Video 3). Dural Flutter (DF), soft and violent can be provoked as additional EPD. Soft DF can be elicited by thumb pressure on water flow at the outer end of the working channel (Supplementary Video 4). DF may be deceptive in cases of obese patients because of epidural fat popping [1,31]. Another conceptual change is to understand that disc prolapse material can have associated end plate cartilage or/and annulus in addition to nucleus fragment. This may have to be removed for complete decompression especially with acute on chronic clinical history. There may be small to big end plate junction failure or posterior rim apophysis fracture [1,32,33]. This may be healed or avulsed or non-united and may necessitate removal when needed. So, a careful assessment of pre-operative MRI is a must. EPD of violent DF can be elicited by a violent cough impulse/deep breathing or any valsalva maneuver. But this method does not hold in case of patients under general anesthesia, and in those cases input from anesthetist with an Ambu bag becomes necessary. Also, to note, Violent Cough or sneeze is now painless unlike pre-operatively, where any valsalva maneuver is usually painful in an LDH (Supplementary Video 2).
Various subtle and obvious clinical changes occur which signals an inferred EPD. A pre-operatively obvious scoliotic list/ tilt/ stoop can correct on operation table itself with subsidence of pain once neural compression is relieved. A changed spasmodic prominence of paraspinal muscles is also observed in many cases. Systemic parameters change and we have consistently observed a fall in heart rate post- decompression after removal of the nociceptive stimulus. Good coordination with the anesthesiologist can help record this finding [34]. Usually, patients of acute, severely painful LDH gets relieved of symptoms and many times goes for a short nap in absence of the severe pain which had given them sleepless previous nights. SLRT (Straight leg raising test) can be elicited by dropping the affected side leg side table of an orthopaedic table or shifting the patient to table side (Figure 11). This can be done supine or lateral as well. This signifies the free sliding of the TNR and can be documented directly. Surgeons must remember that false negative SLRT can be elicited when a chronic disc with a soft disc fragment is operated by PTELD. The focus of the surgery by most surgeon is usually the removal of the soft fragment. Inspite of the adequate removal of the soft fragment, and acute pain relief, the SLRT may not improve due to the bumpy remaining ventral stenosis (Figure 12). False positive free SLR can be elicited in cases of smaller fragments even if they are not removed, under the strong transient action of sensorcaine and washout of the chemical mediators. Epidural Probing (Supplementary Video 5) with a RF probe, hook, dissector, straight probe or steerable probe or articulated hook adds to surgeon’s confidence and confirms epidural clearance. After adequate decompression the articulated hook can be used for the smooth sweeping of the floor of the room that is cleared (i.e., the space ventral to the dura on the annulus and adjoining vertebral body). This also confirms the EPD (Supplementary Video 6, 7). But again, it is inventory based and the extended tactile feedback can be developed by experience only. Usually a Epidural Darks (ED) region is well noticed as empty space many times after the LDH decompression indicating unobstructed epidural space. They are apparent due to decompression and collapsing dura due to irrigating fluid pressure. They start appearing after a decompression indicating EPD but not regularly seen in LDH (Supplementary Video 8, 9). This is usually visible when doing a migrated big LDH removal. A trans-ligamentous sequestration may not show ED. A sub-ligamentous sequestration even after decompression, does not allow the PLL to fall back to its natural position there by preventing typical neural fall back and at times showing false positive ED. Neural tissue visualization is also incomplete in cases of sub ligamentous small protrusions, extrusions and sequestrations. False positive ED is noted in cases of calcified disc adjoining regions even before decompression is completed (Figure 13). Proper in-depth MRI understanding of the patho-anatomy is essential to corroborate these EPD finding. A classification based understanding of ESS is recommended for more specific target identification and execution [1,35].
Instrument tip like a RF probe or hook can be placed and target area location on fluoroscopy can be confirmed (Figure 14). The position of the instrument tip is matched with the location of the pathology on the pre-operative scan to confirm the fact that the surgical decompression was carried out at the correct target pathological area. This maneuver is especially useful in cases with migrated LDH. Recording a video of the entire procedure if feasible or at least the maximum possible EPD’s is recommended by the author for documentation and medico-legal purposes. All the above mentioned EPD are of IO approach. In OI approach also the visualization is similar, but docking is different and maneuverability of the endoscope is limited. The aiming and trajectory have to reach to the head or tail of the fragment. Though after an IO approach burred foraminoplasty can be done to do additional targets of decompression to combine the benefits of IO and OI approaches of PTELD. But the reverse to change from OI to IO approach is technically impossible. In FEE technique the approach is more flatter, visualization is more anatomical and many of the above EPD can be elicited. But, it’s wide spread acceptance is not observed. At the same time the recurrence rates and advanced applications of FEE approach is limited in literature [36,37].
2) EPD in PTELD for StenosisThe detailed techniques of execution of stenotic decompression can be referred to in previous literature and is not the focus of present study [1]. Many suggested EPD, found in literature search, are there in transforaminal endoscopic lateral recess decompression, lumbar foraminotomy and ventral decompression (Table 1). The commonest pathology in degenerative stenosis is hypertrophy of the SAP (superior articular process), LF (ligamentum flavum) hypertrophy, disc space settling (annulus buckling) with or without LDH, osteophyte formation and associated dynamic or static translation. As a result, the TNR is compressed in cases of lateral recess stenosis (LRS) and the exiting nerve root is compressed in the foraminal stenosis (FS) [38,39]. LSS according to pathological zones is classified into three categories: central LSS, LRS and FS. PTELD can be suitable for the treatment of the LRS/FS by resection of the hypertrophied SAP [40-42]. Reaching to the central dorsal aspect is difficult by PTELD. Though tricky, indirect decompression by removing the ventral upper endplate spur of lower vertebra can enlarge the central canal as well [41]. In FS, the focus is on the cranial foramen and removal of the tip of SAP, capsule and LF. Visualization of the pulsatile exiting nerve root is the EPD. For the LRS, the caudal foramen is focussed, and additional adjoining pedicle removal is needed many times in addition to sculpting of SAP, inferior articular process, LF and capsule. Visualisation of the entire pulsatile TNR on ventral, lateral and dorsal aspect confirms the EPD (Supplementary Video 9) [43]. For central LSS, or unilateral ventral stenosis, ventral decompression by removal of the LDH, buckling annulus (which is usually hardened or calcified) and removal of the superior vertebral end plate spur of the inferior vertebra is done (Supplementary Video 10). Visualisation of the entire pulsatile TNR and dural sac affirms the EPD. For bilateral symptomatic cases further flattening of the endoscope trajectory and ventral decompression of contralateral traversing root and visualization is to be done [44]. In cases with a high degree of stenosis, bilateral symptoms, conus/cauda equina syndrome, bilateral transforaminal endoscopy with two working ports ensures adequate EPD (Figure 15) [1]. Usually a epidural dark region is well noticed many times after the decompression indicating unobstructed epidural space (Supplementary Video 8, 9). This can be ventral or dorsal. False positive epidural dark are visible at some times especially at elevated dura junction of calcified disc/end plate spur and represent dead spaces due to chronicity (Figure 13).
3. Post-operative AssessmentSLRT can be utilized pre-operatively with diagnostic implications and intra-operatively/post-operatively to confirm the EPD [45,46]. There may be a component of hamstring tightness [47] which may remain; however, the characteristic radicular pain subsides [48]. It is important to note that this is a “passive” SLRT [49] to be performed by the surgeon only and not to be done by the patient on his own.
Post-operative VAS (visual analogue scale) score can be compared with the pre-operative baseline [50]. This assumes importance as the PTELD elicited VAS is very specific and not confounded by surgical site pain as in COSS [51]. PROM (Patient Reported Outcome Measures) evaluations follow few weeks to months and objectifies the recovery and optimum decompression achieved. But, patient satisfaction Index or other Likart scales can be taken immediately to quantify the subjective outcomes [51,52].
The incidence of scoliotic list has been reported to range between 8.9% to 18% in patients with LDH who have undergone a discectomy [53]. Disappearance of a pre-operative list either intra-operatively on table or post operatively is observed and confirms the adequacy of decompression.
We routinely do immediate post-operative MRI (PMRI) combined with Magnetic Resonance Myelography (MRM) [54,55] to objectively assess the extent of decompression in all patients. PMRI (Figure 16) is best confirmatory for all PTELD. A resolved MRM block also facilitates documentation of the EPD. Myelo-block after decompression are visible in many cases because of unaddressed endplate spur ventral stenosis (Figure 12). Even with multiple EPD achieved per-operatively and annular flap mobility, myelo-block may be there (False positive) (Figure 17). Pseudobulges are noted in some cases. This is very important to record in consultation with radiologist as it can invite medicolegal problems especially in profound deficits, despite an optimal EPD achieved [1,56].
4. Future DirectionsIntra-operative ultrasound [57,58] guided PTELD is a new adjunct that gives radiation free real time guidance. Ultrasound has been utilized in COSS, not only for identification of spinal pathologies operated, but also to confirm the degree of decompression of the neural elements [59-61]. In PTELD the need is an appropriate probe, engineered to go through the working sheath. We can also use epiduroscope/bronchoscope through the working cannula to visualize the EPD (Figure 18). This can be utilized when highly migrated fragments are there, which can be fragmented. UBS (ultrasonic bone scalpel) in COSS shows several advantages including decreased risk of mechanical injury, reduced thermal injury, and reduction in osseous bleeding, which improves visibility in the surgical field and provides significant reduction in surgical time [62-65]. Given the precision offered by UBS, and the fact that PTELD is such a selective, targeted procedure, the day when UBS will be introduced in ESS is not far away. Navigation is another development in spine surgery and PTELD [66,67]. It will enhance the surgeon’s ease of learning and performing PTELD and likely save radiation years of surgeon [68]. When combined with intra-operative CT scan based navigation, EPD can be confirmed when PTELD is applied for bony stenosis. Possibility of endoscopic vision with projection of 3D digital hologram defining the EPD can be planned pre-operatively and confirmed per-operatively, based on interactive user interface on an head mounted device based navigation [69,70]. Robotic spine systems are rapidly changing the landscape of modern surgery. Once appropriate user interfaces are in play, RSS will be useful for decompression procedures as well [71,72]. Again, the EPD can be programmed pre-operatively and reached per-operatively using external image guidance and surgeon inputs [73-75]. Choi et al. [76] used a specially designed fluoroscope with MRI-equipped operative suite (XMR) which would objectively confirm the EPD per-operatively thereby reducing the chances of failures [74-76].
Limitations exists to the authenticity of observations and write-up of this article. This is a narrative review and not a systematic review or a meta-analysis [77]. The search was restricted to English literature in PubMed database only. Hence, there are literature focussed on the topic that may have been missed. This article contains information, observations, personal views, suggestions and experience-based statements which needs to be validated by more experienced pioneers’ world over before following it as a thumb rule. Sensitivity and specificity validation needs to be done in series of consecutive patients. But each of the EPD has been defined, objectified and supported with an image, illustration or video. However, the figures and videos are focussed specifically on EPD and this article is targeted towards surgeons already performing PTELD. Detailed step by step videos/technique description is not included as this was not the prime focus of this article. But we believe that our attempt is justified with the fact that in COSS and MIS surgeries also the EPD are not yet fully defined. PTELD technique has been proven through randomized trials and meta-analyses as an excellent alternative surgical option to MIS and COSS [78-81]. Defining and reaching the EPD will ensure reduced failures and in-turn improve the wider acceptability of this procedure [82].
CONCLUSIONThe EPD is variable and depends on numerous factors, not all signs may be elicited in every case and may change with surgeon experience. The ability to recognize the EPD is the crux for a successful complete decompression. Though debatable, maximum EPD’s should be aimed in every surgery to avoid failures.
Supplementary MaterialsSupplementary Video 1.Major Fragment Retrieval (MFR) (https://doi.org/10.21182/jmisst.2022.00444.v001).
Supplementary Video 2.(A) Illustrative prone anatomy-patient orientation with area of docked endoscope tip (red outline) at para-central location. (B) Showing a pulsatile, moving dural sac with opposite annular fissure visualisation (AFV) and probing with a flexible ball tip to confirm End point decompression (EPD) of the dural sac and the opposite side traversing nerve root (TNR). Also to note are annular flap mobility (superior and inferior both) (AFM). Violent dural flutter also is elicited with deep breathing (https://doi.org/10.21182/jmisst.2022.00444.v002).
Supplementary Video 3.(A) Illustrative prone anatomy-patient orientation with area of docked endoscope tip (red outline) at supra-pedicular lateral location, having removed ventro-lateral part of superior facet by blind foraminoplasty. (B) After a transforaminal “Outside In” (OI) approach (Oblique view is Obvious) decompression of a migrated fragment is achieved with visualised ligamentam flavum curtain (black*), ventral annular tear (orange*) and decompressed traversing nerve root (ventral and lateral aspect). The nerve root is pulsatile and matching with the heart rate, indicating End point decompression (EPD) (https://doi.org/10.21182/jmisst.2022.00444.v003).
Supplementary Video 4.Video in video of the external generated fluid ripples that get reflected inside to elicit dural flutter (DF) which is a provocative end point Decompression (EPD). Even more violent EPD can be generated by asking patient to cough or with a Valsalva maneuver (VM) (https://doi.org/10.21182/jmisst.2022.00444.v004).
Supplementary Video 5.(A) Illustrative prone anatomy-patient Orientation with area of docked endoscope tip (red outline) at para-central location after an “Inside Out” (IO) approach for paracentral small disc protrusion. Lateral Recess visualisation of decompressed traversing nerve root (TNR) is not clear as posterior longitudinal ligament (PLL) and annulus both are not cut. So this is an inferred End-point decompression (EPD) with use of the radio frequency probe wanding and feeling below and above the Annulo-PLL tissue (https://doi.org/10.21182/jmisst.2022.00444.v005).
Supplementary Video 6.(A) Illustrative prone anatomy-patient Orientation with area of docked endoscope tip (red outline) at inferior left foraminal para-central location in an “Inside out” (IO) approach. After removal of a trans-ligamentous (PLL: posterior longitudinal ligament) extruded central disc fragment. Video (B) Smooth Sweeping of the floor (SSF). Pulsations and dural flutter are present to confirm End point decompression (EPD) as well. The fluffy annulus tear with PLL appears compressive. But, actually it’s mobile and does not contribute to any stenosis (https://doi.org/10.21182/jmisst.2022.00444.v006).
Supplementary Video 7.Fluoroscopy live image intensifier video showing the articulated hook used for the floor (SSF) as End point decompression (EPD). Video 20/8: (A) Illustrative prone anatomy-patient orientation with area of docked endoscope tip (red outline) supra-pedicular lateral location, having removed ventro-lateral part of superior facet and part of pedicle by endoscopic burred-visualised foraminoplasty. (B) After a transforaminal “Outside In” (OI) approach decompression of a migrated fragment is achieved with visualised decompressed traversing nerve root (TNR) (ventral and lateral aspect), is pulsatile and matching with heart rate. Additionally, Epidural darks (ED) is visible ventrally indicating End point decompression (EPD) (https://doi.org/10.21182/jmisst.2022.00444.v007).
Supplementary Video 8.(A) Illustrative prone anatomy-patient orientation with area of docked endoscope tip (red outline) supra-pedicular lateral location, having removed ventro-lateral part of superior facet and part of pedicle by endoscopic burred-visualised foraminoplasty. (B) After a transforaminal “Outside In” (OI) approach decompression of a migrated fragment is achieved with visualised decompressed traversing nerve root (TNR) (ventral and lateral aspect), is pulsatile and matching with heart rate. Additionally, Epidural darks (ED) is visible ventrally indicating End point decompression (EPD) (https://doi.org/10.21182/jmisst.2022.00444.v008).
Supplementary Video 9.(A) Illustrative prone anatomy-patient Orientation with area of docked endoscope tip (red outline) at para-central location after foraminoplasty. The ligaments flavum (LF) is also removed dorsally (B) Lateral Recess visualisation of decompressed traversing nerve root (TNR) dorsal as well as ventral: Dural Flutter (DF), epidural darks (ED) clearly confirms the End point decompression (EPD) in a lateral recess stenosis (LRS). Tuft of capsule of facet joint and LF in superior foramen, not causing any TNR compression is left as it is (https://doi.org/10.21182/jmisst.2022.00444.v009).
Supplementary Video 10.(A) Illustrative prone anatomy-patient orientation with area of docked endoscope tip (red outline) inferior foraminal para-central location (B). The EPD (End point decompression) in ventral stenosis due to bony spurs is a very small focussed area following removal of the ventral stenosis with burrs and osteotome, showing the fluttering pulsatile traversing nerve root (TNR) (https://doi.org/10.21182/jmisst.2022.00444.v010).
Figure 2.(A–C) MRI of L3-4 para-central location low migrated LDH on left side. (D, E) Superimposed traced fragment (Red) in a antero-posterior and lateral radiograph. (F, G) The position of the endoscope tip and the articulated hook checked on C-arm for target area location assessment. End point decompression (EPD) is confirmed after fragment retrieval and inferred with target area location and re-affirmed with a post-operative MRI later. 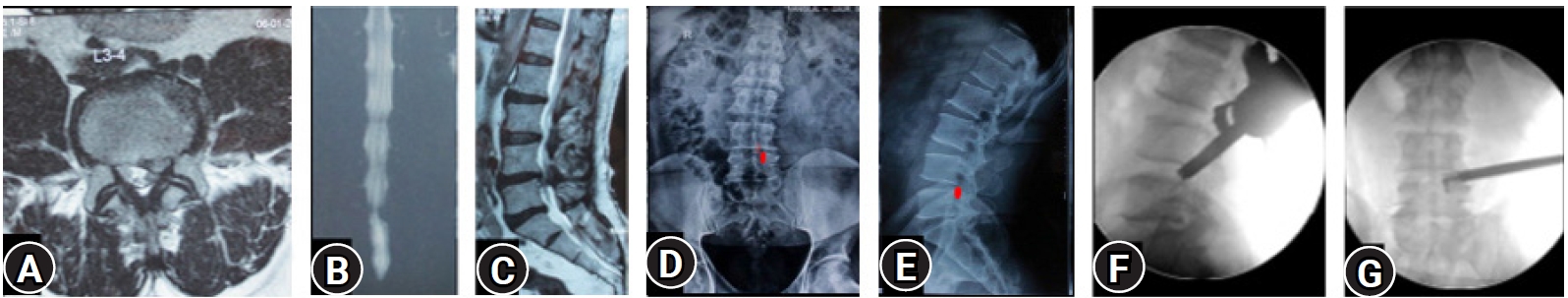
Figure 3.(A) Illustrative prone anatomy-patient orientation with area of docked endoscope tip (red outline) para-central location. (B) At the start of the left side approach to L4-5, Endoscopic “Inside out” epidural view showing starting endoscopic view with the nucleus pulposus fragment, lying just ahead after having cut the near side annular anchorage. (C) After the completion of Transforaminal, Full Endoscopic Spine surgery (ESS) the End Point Decompression (EPD) clearly showing the neural fall back with epidural vessels (black*), into the space created by removal of the disc fragment. Opposite annular fissure visualisation (orange*) at the depth of the annular tear which was on the other side of the cut side annular anchorage. 
Figure 4.(A) Illustrative prone anatomy-patient orientation with area of docked endoscope tip (red outline) at para-central location. (B) Epidural fat pop-out and epidural vessels that gets visualized in obese patients rather than neural fall back. 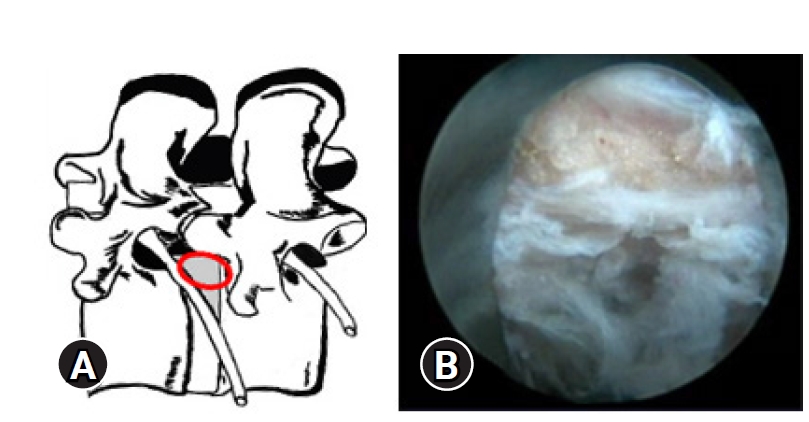
Figure 5.After the major fragment retrieval, habit of eyeballing the size of fragment removed and matching it with an extruded or sequestrated fragment size on MRI should be the dictum. Variable colours are valuable to identify the major fragment. Red area shows the epidural outer surface, while yellowish area is unexposed part, though degenerated. Some times a more whitish coloured area is noted and is the most inside part of the major fragment within the disc space confines itself. 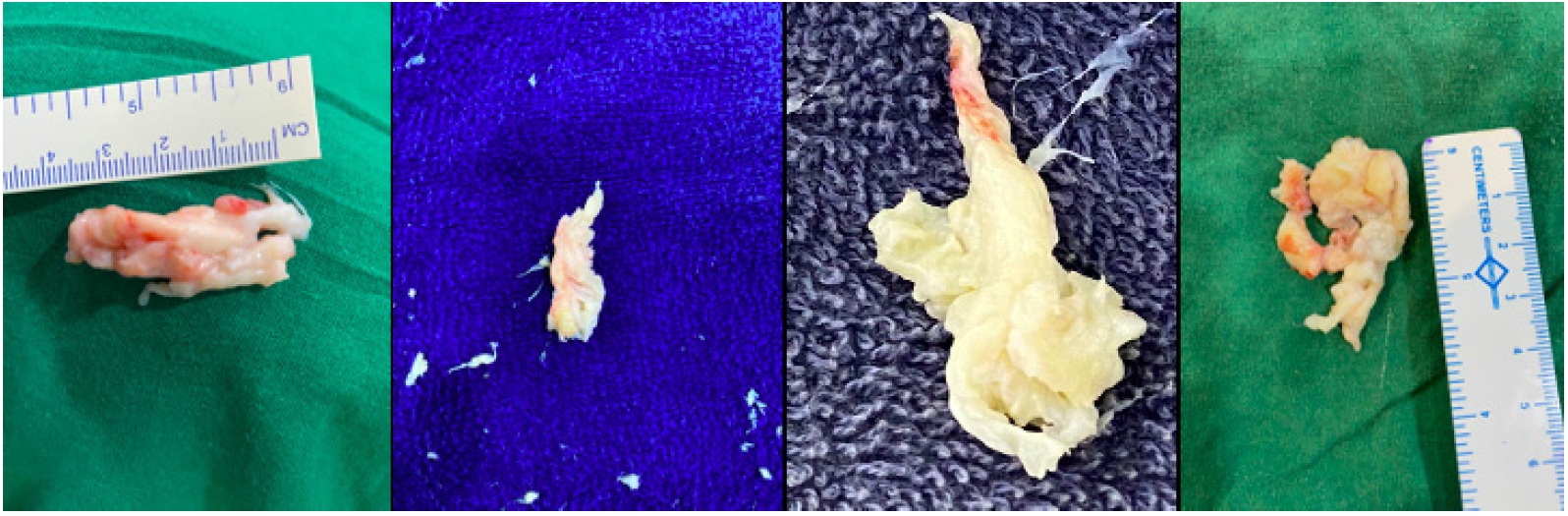
Figure 7.Illustrative image showing the annular tear through which the nucleus has prolapsed. After PTELD (removal of fragment), the Opposite annular fissure visualization and annular flaps mobility are EPD’s (End Point of Decompression) 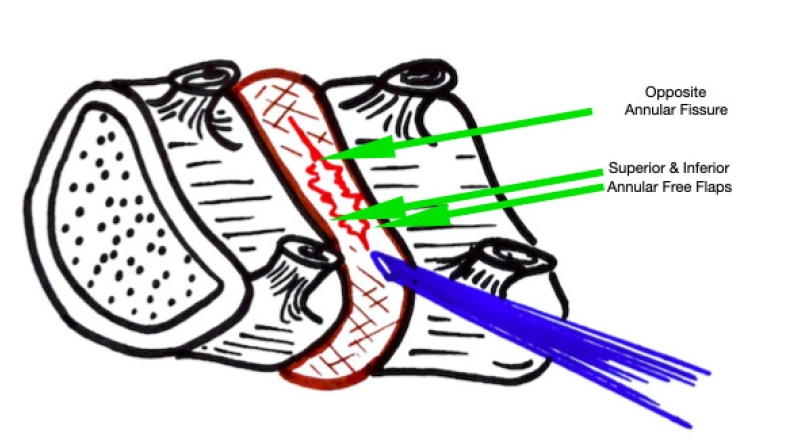
Figure 8.Eye balling the size of fragment and comparing with the MRI. Previous two level operated Open surgery patient with a new L2-3-disc herniation (A, B), with a matched fragment en-masse removal (C). 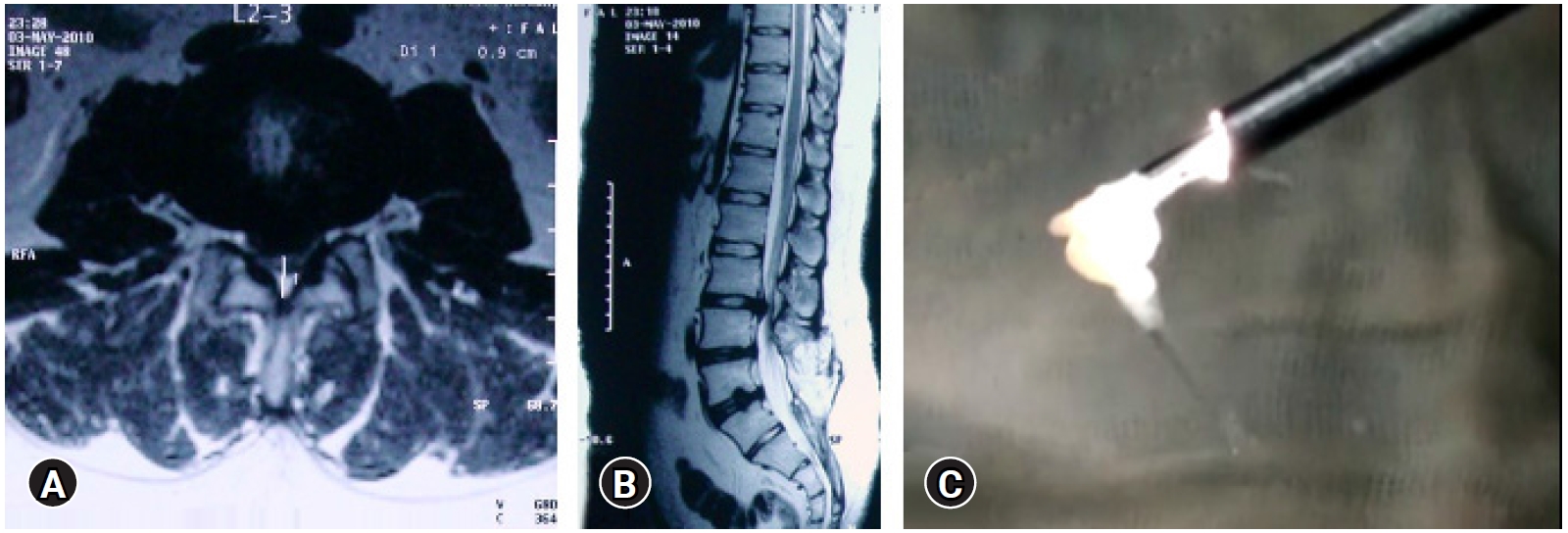
Figure 9.(A, C) An operated case of up-migrated disc prolapse (L 4-5: Right side) by transforaminal “Inside Out” approach. (B, D) Near complete decompression in the Post-operative confirmatory MRI showing the achieved decompression. A small residual asymptomatic fragment remains and at 6 years’ post-operative the outcome is excellent. 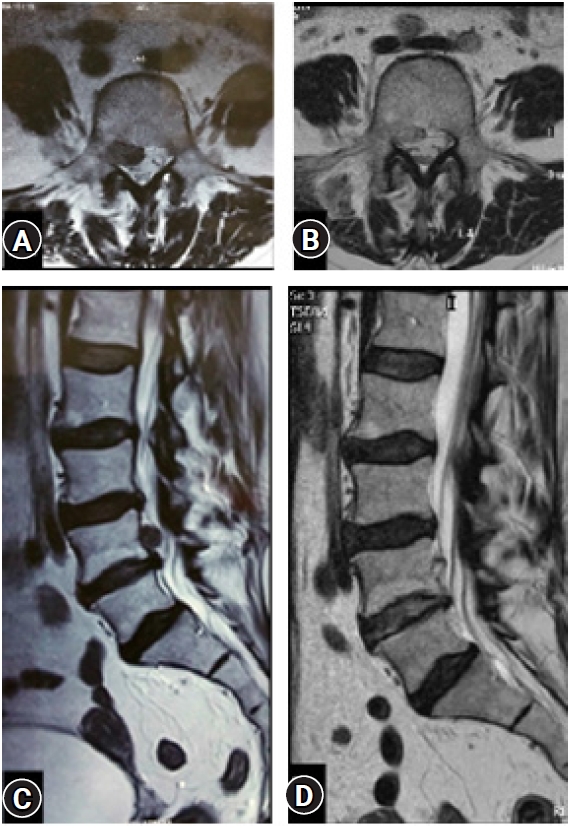
Figure 10.Illustration of a calcified disc, superior end plate spur of inferior vertebra, causing hard ventral lateral recess stenosis. Similar spur may exist in upper or lower endplate and even bilaterally. 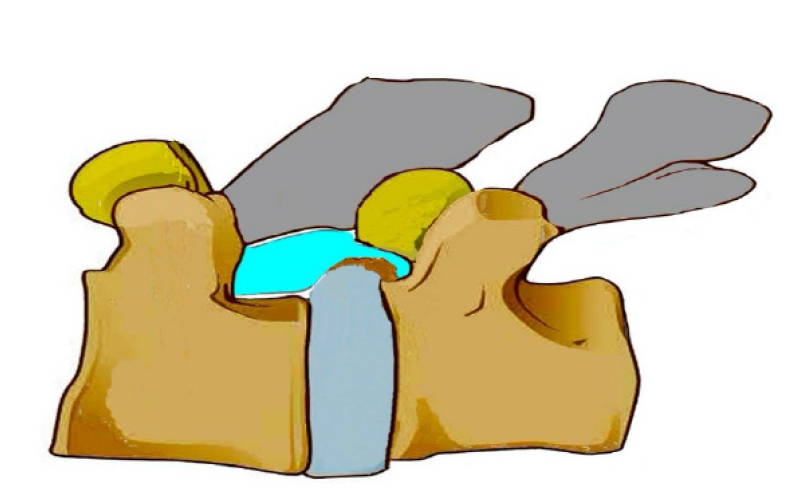
Figure 11.Straight leg raising test elicited in prone position on table (per-operative) which if free in comparison to the restricted pre-operative test, suggesting inferred EPD (End Point Decompression). 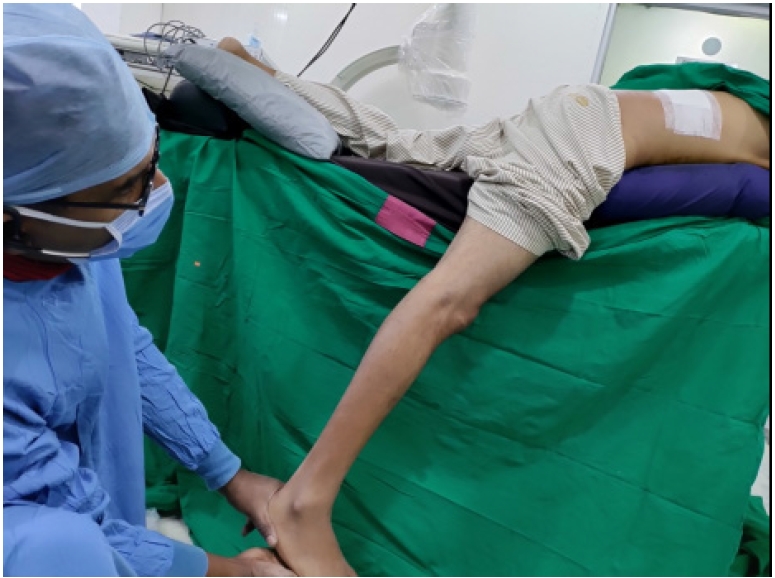
Figure 12.(A) Showing a calcified end plate spur causing ventral stenosis with a small disc prolapse that causes an acute presentation. (B) Even after removal of the disc fragment by PTELD, the ventral stenosis remains. In this case acute symptoms may resolve but SLRT restriction may remain in moderation. The myelo-block in myelogram can also persist. 
Figure 13.Black shadow in ventral epidural space adjoining the discogenic compression, suggestive of false positive Epidural darks. These are dead spaces and are present in severe ventral stenosis. 
Figure 14.The position of the endoscope tip and the articulated grasper can be checked on fluoroscopy for target location assessment target area location. (A, B) In case of a highly migrated disc prolapse tackled with a transforaminal “Outside In” approach, End-point decompression (EPD) confirmed after major fragment retrieval and inferred by reach and position of the instrument tip. 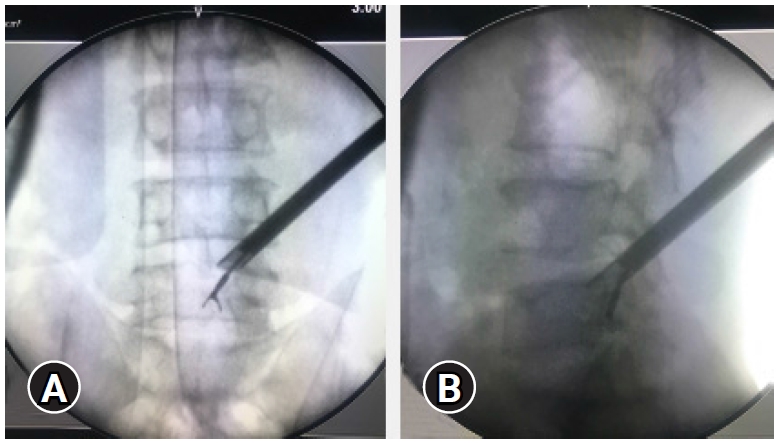
Figure 15.(A) Image intensifier view of a bilateral transforaminal endoscopy with two working ports. (B) The one side view showing the opposite working sheath with removed ventral stenosis and with neural fall back suggesting the EPD (End point decompression). (C, D) CT scan and MRI showing calcified ventral disc and posterior longitudinal ligament in a case for Conus syndrome L1-L2 with paraparesis. (E) Post-operative MRI conforming the extend of decompression. Patient recovered completely in two weeks’ time and 7 years follow-up. 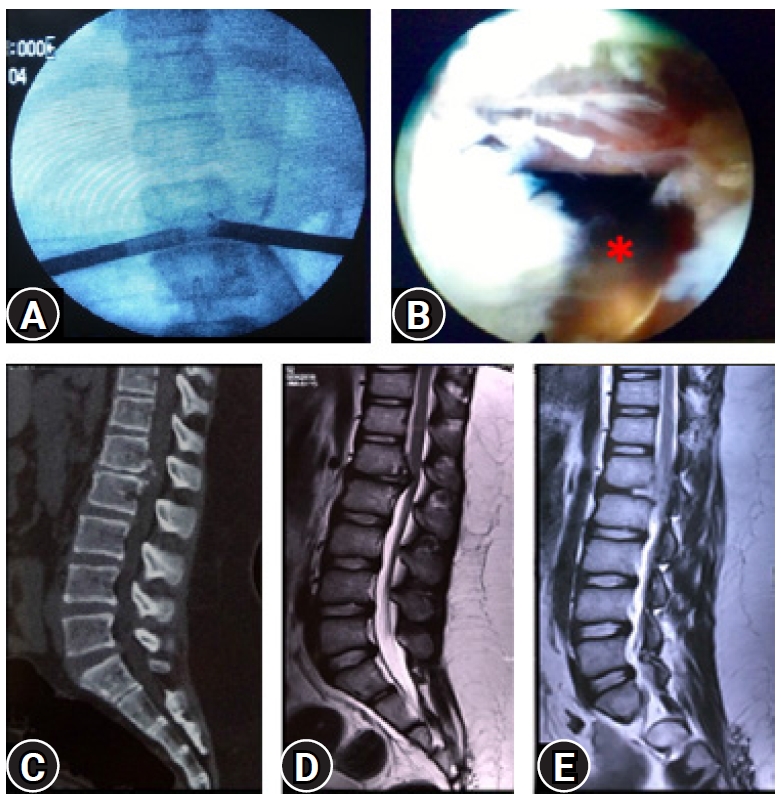
Figure 16.Post-operative confirmatory MRI (C, D) of a case with extra-foraminal disc prolapse (Yellow arrow) (A, B) showing complete decompression and documentation. 
Figure 17.False Positive MRI myelo-block. In spite of removal of the culprit fragment and EPD achieved with soft dural flutter, opposite annular fissure visualization), epidural probing, and annular flap mobility, a pseudobulge is visible on the post-operative MRI. The patient was non-symptomatic and excellent clinical outcome. 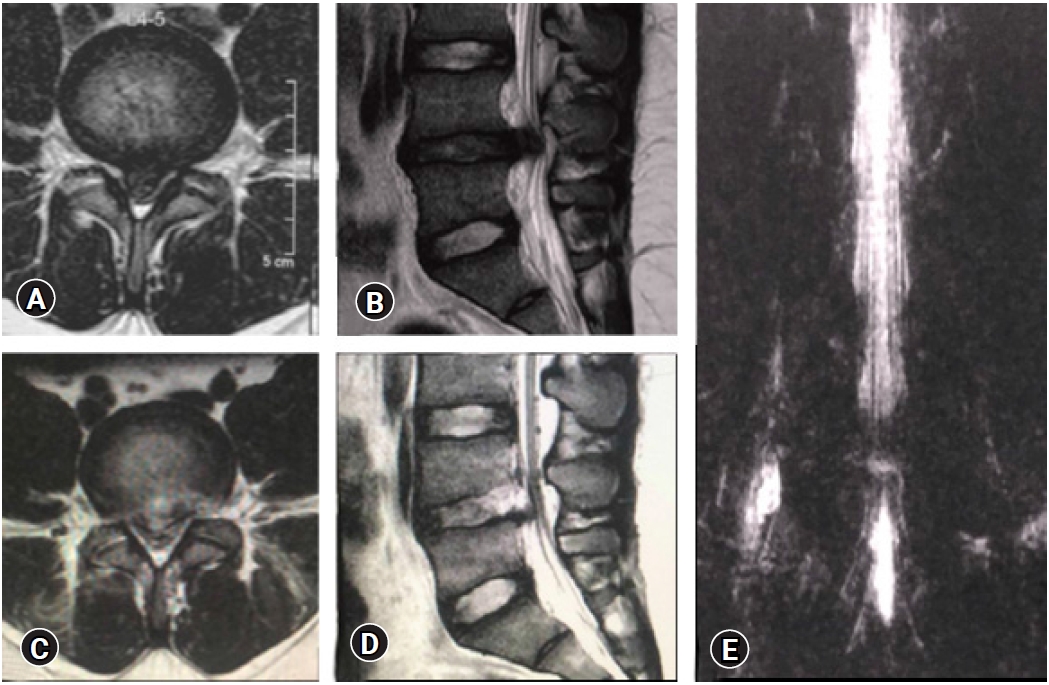
Table 1.EPD (end-point of decompression) in transforaminal endoscopy defined in PubMed literature search
Table 2.Authors recommended EPD (End Point Decompression) REFERENCES1. Krishnan A, Kim HS, Raj A, Dave BR. Expanded indications of full endoscopic spine sugery. J Minim Invasive Spine Surg Tech 2021;6:S130–S156.
2. Choi G, Pophale CS, Patel B, Uniyal P. Endoscopic spine surgery. J Korean Neurosurg Soc 2017;60:485–497.
3. Telfeian AE, Veeravagu A, Oyelese AA, Gokaslan ZL. A brief history of endoscopic spine surgery. Neurosurg Focus 2016;40:E2.
4. Moon ASM, Rajaram Manoharan SR. Endoscopic spine surgery: current state of art and the future perspective. Asian Spine J 2018;12:1–2.
5. Kim M, Kim HS, Oh SW, Adsul NM, Singh R, Kashlan ON, et al. Evolution of spinal endoscopic surgery. Neurospine 2019;16:6–14.
6. Lee S, Kim SK, Lee SH, Kim WJ, Choi WC, Choi G, et al. Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J 2007;16:431–437.
7. Ahn Y, Jang IT, Kim WK. Transforaminal percutaneous endoscopic lumbar discectomy for very high-grade migrated disc herniation. Clin Neurol Neurosurg 2016;147:11–17.
8. Choi KC, Lee DC, Shim HK, Shin SH, Park CK. A strategy of percutaneous endoscopic lumbar discectomy for migrated disc herniation. World Neurosurg 2017;99:259–266.
9. Krishnan A, Barot MP, Dave BR, Bang P, Devanand D, Patel D, et al. Percutaneous transforaminal endoscopic decompression and cageless percutaneous bone graft transforaminal lumbar interbody fusion: a feasibility study. J Orthop Allied Sci 2018;6:S21–S27.
10. Krishnan A, Barot MP, Dave BR, Bang P, Devanand D, Patel D, et al. Percutaneous transforaminal endoscopic discectomy and drainage for spondylodiscitis: a technical note and review of literature. J Orthop Allied Sci 2018;6:S16–S20.
11. Zuckerman SL, Laufer I, Sahgal A, Yamada YJ, Schmidt MH, Chou D, et al. When less is more: the indications for MIS techniques and separation surgery in metastatic spine disease. Spine (Phila Pa 1976) 2016;41 Suppl 20:S246–S253.
12. Sharma SB, Lin GX, Jabri H, Sidappa ND, Song MS, Choi KC, et al. Radiographic and clinical outcomes of huge lumbar disc herniations treated by transforaminal endoscopic discectomy. Clin Neurol Neurosurg 2019;185:105485.
13. Kim HS, Sharma SB, Wu PH, Raorane HD, Adsul NM, Singh R, et al. Complications and limitations of endoscopic spine surgery and percutaneous instrumentation. Indian Spine J 2020;3:78–85.
14. Sood A, Ghosh AK. Literature search using PubMed: an essential tool for practicing evidence-based medicine. J Assoc Physicians India 2006;54:303–308.
15. Gadiya AD, Borde MD, Kumar N, Patel PM, Nagad PB, Bhojraj SY. Response to: analysis of functional and radiological outcome following lumbar decompression without fusion in patients with degenerative lumbar scoliosis. Asian Spine J 2020;14:588–589.
16. Gupta S, Marathe N, Chhabra HS, Destandau J. Long-term functional outcomes of endoscopic decompression with Destandau technique for lumbar canal stenosis. Asian Spine J 2021;15:431–440.
17. Lin GX, Huang P, Kotheeranurak V, Park CW, Heo DH, Park CK, et al. A systematic review of unilateral biportal endoscopic spinal surgery: preliminary clinical results and complications. World Neurosurg 2019;125:425–432.
18. Nam HGW, Kim HS, Lee DK, Park CK, Lim KT. Percutaneous stenoscopic lumbar decompression with paramedian approach for foraminal/extraforaminal lesions. Asian Spine J 2019;13:672–681.
19. Patgaonkar P, Marathe NA, Goyal V, Agrawal U, Patel V. Adolescent lumbar disc herniation with a peculiar gait pattern managed by transforaminal endoscopic spine surgery. J Orthop Case Rep 2020;10:93–96.
20. Elsig JP, Kaech DL. Imaging-based planning for spine surgery. Minim Invasive Ther Allied Technol 2006;15:260–266.
21. Yoshinari H, Tezuka F, Yamashita K, Manabe H, Hayashi F, Ishihama Y, et al. Transforaminal full-endoscopic lumbar discectomy under local anesthesia in awake and aware conditions: the inside-out and outside-in techniques. Curr Rev Musculoskelet Med 2019;12:311–317.
22. Lee SG, Ahn Y. Transforaminal endoscopic lumbar discectomy: basic concepts and technical keys to clinical success. Int J Spine Surg 2021;15(suppl 3):S38–S46.
23. Lewandrowski KU. The strategies behind “inside-out” and “outside-in” endoscopy of the lumbar spine: treating the pain generator. J Spine Surg 2020;6:S35–S39.
24. Lewandrowski KU, Yeung A. Lumbar endoscopic bony and soft tissue decompression with the hybridized inside-out approach: a review and technical note. Neurospine 2020;17:S34–S43.
25. Komp M, Hahn P, Ozdemir S, Merk H, Kasch R, Godolias G, et al. Operation of lumbar zygoapophyseal joint cysts using a full-endoscopic interlaminar and transforaminal approach: prospective 2-year results of 74 patients. Surg Innov 2014;21:605–614.
26. Gorth DJ, Ottone OK, Shapiro IM, Risbud MV. Differential effect of long-term systemic exposure of TNFα on health of the annulus fibrosus and nucleus pulposus of the intervertebral disc. J Bone Miner Res 2020;35:725–737.
27. Di Martino A, Merlini L, Faldini C. Autoimmunity in intervertebral disc herniation: from bench to bedside. Expert Opin Ther Targets 2013;17:1461–1470.
28. Wu C, Lee CY, Chen SC, Hsu SK, Wu MH. Functional outcomes of full-endoscopic spine surgery for high-grade migrated lumbar disc herniation: a prospective registry-based cohort study with more than 5 years of follow-up. BMC Musculoskelet Disord 2021;22:58.
29. Kim IS, Kim KH, Shin SW, Kim TK, Kim JI. Indigo carmine for the selective endoscopic intervertebral nuclectomy. J Korean Med Sci 2005;20:702–703.
30. Yeung AT. The evolution and advancement of endoscopic foraminal surgery: one surgeon’s experience incorporating adjunctive techologies. SAS J 2007;1:108–117.
31. Wang YP, Zhang W, An JL, Zhang J, Bai JY, Sun YP. Evaluation of transforaminal endoscopic discectomy in treatment of obese patients with lumbar disc herniation. Med Sci Monit 2016;22:2513–2519.
32. Krishnan A, Patel JG, Patel DA, Patel PR. Fracture of posterior margin of lumbar vertebral body. Indian J Orthop 2005;39:33–38.
33. Rajasekaran S, Bajaj N, Tubaki V, Kanna RM, Shetty AP. ISSLS prize winner: the anatomy of failure in lumbar disc herniation: an in vivo, multimodal, prospective study of 181 subjects. Spine (Phila Pa 1976) 2013;38:1491–1500.
34. Krishnan A, Degulmadi D, Ranjan R, Mayi S, Nitherwal N, Reddy L, et al. Hemodynamic neuromonitoring, a proposed spino-cardiac protective reflex: prospective study in 200 patients of lumbar surgery. Back Bone: Spine J 2021-2022;2:71–78.
35. Hofstetter CP, Ahn Y, Choi G, Gibson JNA, Ruetten S, Zhou Y, et al. AOSpine consensus paper on nomenclature for working-channel endoscopic spinal procedures. Global Spine J 2020;10:111S–121S.
36. Ruetten S, Komp M, Godolias G. An extreme lateral access for the surgery of lumbar disc herniations inside the spinal canal using the full-endoscopic uniportal transforaminal approach-technique and prospective results of 463 patients. Spine (Phila Pa 1976) 2005;30:2570–2578.
37. Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 2008;33:931–939.
38. Terai T. Outside-in Direct Fragmentectomy of TELD after Foraminoplasty. In: Sairyo K, editor. Transforaminal Full-endoscopic Lumbar Surgery under the Local Anesthesia. Singapore: Springer; 2021. p. 37–45.
39. Chen C, Ma X, Zhao D, Yang H, Xu B, Wang Z, et al. Full endoscopic lumbar foraminoplasty with periendoscopic visualized trephine technique for lumbar disc herniation with migration and/or foraminal or lateral recess stenosis. World Neurosurg 2021;148:e658–e666.
40. Kim JY, Kim HS, Jeon JB, Lee JH, Park JH, Jang IT. The novel technique of uniportal endoscopic interlaminar contralateral approach for coexisting L5-S1 lateral recess, foraminal, and extraforaminal stenosis and its clinical outcomes. J Clin Med 2021;10:1364.
41. Sairyo K, Higashino K, Yamashita K, Hayashi F, Wada K, Sakai T, et al. A new concept of transforaminal ventral facetectomy including simultaneous decompression of foraminal and lateral recess stenosis: technical considerations in a fresh cadaver model and a literature review. J Med Invest 2017;64:1–6.
42. Yamashita K. Full-endoscopic Lateral Recess Decompression (Ventral Facetectomy). In: Sairyo K, editor. Transforaminal Full-endoscopic Lumbar Surgery under the Local Anesthesia. Singapore: Springer; 2021. p. 63–67.
43. Krishnan A, Kulkarni M, Singh M, Reddy C, Mayi S, Devanand D, et al. Trans-foraminal endoscopic uniportal decompression in degenerative lumbar spondylolisthesis: a technical and case report. Egypt J Neurosurg 2019;34:34.
44. Choi KC, Shim HK. Full Endoscopic Approach with Foraminoplasty. In: Kim HS, Mayer M, Heo DH, Park CW, editors. Advanced Techniques of Endoscopic Lumbar Spine Surgery. Singapore: Springer; 2020. p. 103–113.
45. Kumar N, Wijerathne SI, Lim WW, Barry TW, Nath C, Liang S. Resistive straight leg raise test, resistive forward bend test and heel compression test: novel techniques in identifying secondary gain motives in low back pain cases. Eur Spine J 2012;21:2280–2286.
46. Ahn JS, Lee JK, Kwon YS, Hong UP. Intraoperative straight leg raising test during arthroscopic microdiscectomy. J Korean Soc Spine Surg 2003 10:25–29. Korean.
47. Atalay A, Akbay A, Atalay B, Akalan N. Lumbar disc herniation and tight hamstrings syndrome in adolescence. Childs Nerv Syst 2003;19:82–85.
48. Samolsky Dekel BG, Sorella MC, Vasarri A, Melotti RM. Reliability of the buttock applied strain test to diagnose radicular pain in patients with low back pain. Pain Pract 2020;20:829–837.
49. Rebain R, Baxter GD, McDonough S. A systematic review of the passive straight leg raising test as a diagnostic aid for low back pain (1989 to 2000). Spine (Phila Pa 1976) 2002;27:E388–E395.
50. Knop C, Oeser M, Bastian L, Lange U, Zdichavsky M, Blauth M. Entwicklung und Validierung des VAS-Wirbelsäulenscores [Development and validation of the Visual Analogue Scale (VAS) Spine Score]. Unfallchirurg 2001 104:488–497. German.
51. Gadjradj PS, van Tulder MW, Dirven CM, Peul WC, Harhangi BS. Clinical outcomes after percutaneous transforaminal endoscopic discectomy for lumbar disc herniation: a prospective case series. Neurosurg Focus 2016;40:E3.
52. Gadjradj PS, Harhangi BS, Amelink J, van Susante J, Kamper S, van Tulder M, et al. Percutaneous transforaminal endoscopic discectomy versus open microdiscectomy for lumbar disc herniation: a systematic review and meta-analysis. Spine (Phila Pa 1976) 2021;46:538–549.
53. Suk KS, Lee HM, Moon SH, Kim NH. Lumbosacral scoliotic list by lumbar disc herniation. Spine (Phila Pa 1976) 2001;26:667–671.
54. Leonardi MA, Zanetti M, Saupe N, Min K. Early postoperative MRI in detecting hematoma and dural compression after lumbar spinal decompression: prospective study of asymptomatic patients in comparison to patients requiring surgical revision. Eur Spine J 2010;19:2216–2222.
55. Floris R, Spallone A, Aref TY, Rizzo A, Apruzzese A, Mulas M, et al. Early postoperative MRI findings following surgery for herniated lumbar disc. Acta Neurochir (Wien) 1997;139:169–175.
56. Namboothiri S, Gore S, Veerasekhar G. Treatment of low back pain by treating the annular high intensity zone (HIZ) lesions using percutaneous transforaminal endoscopic disc surgery. Int J Spine Surg 2018;12:388–392.
57. Ganau M, Syrmos N, Martin AR, Jiang F, Fehlings MG. Intraoperative ultrasound in spine surgery: history, current applications, future developments. Quant Imaging Med Surg 2018;8:261–267.
58. Harel R, Knoller N. Intraoperative spine ultrasound: application and benefits. Eur Spine J 2016;25:865–869.
59. Vasudeva VS, Abd-El-Barr M, Pompeu YA, Karhade A, Groff MW, Lu Y. Use of intraoperative ultrasound during spinal surgery. Global Spine J 2017;7:648–656.
60. Prada F, Vetrano IG, Filippini A, Del Bene M, Perin A, Casali C, et al. Intraoperative ultrasound in spinal tumor surgery. J Ultrasound 2014;17:195–202.
61. Yan CX, Goulet B, Pelletier J, Chen SJ, Tampieri D, Collins DL. Towards accurate, robust and practical ultrasound-CT registration of vertebrae for image-guided spine surgery. Int J Comput Assist Radiol Surg 2011;6:523–537.
62. Dave BR, Degulmadi D, Dahibhate S, Krishnan A, Patel D. Ultrasonic bone scalpel: utility in cervical corpectomy. A technical note. Eur Spine J 2019;28:380–385.
63. Krishnan A, Samal P, Mayi S, Degulmadi S, Rai RR, Dave B. Thoracic spine stenosis: does ultrasonic osteotome improve outcome in comparison to conventional technique. Malays Orthop J 2021;15:62–69.
64. Dave BR, Krishnan A, Rai RR, Degulmadi D, Mayi S, Gudhe M. The effectiveness and safety of ultrasonic bone scalpel versus conventional method in cervical laminectomy: a retrospective study of 311 patients. Global Spine J 2020;10:760–766.
65. Krishnan A, Patil S, Reddy C, Mayi S, Degulmadi D, Rai RR, et al. Ventral sculpting decompression: a novel bone scalpel-based technique in thoracic ventral stenosis/kyphosis with myelopathy. Egypt J Neurosurg 2020;354:4.
66. Sangondimath G, Mallepally AR, Marathe N, Mak KC, Salimath S. Degenerative cervical myelopathy: recent updates and future directions. J Clin Orthop Trauma 2020;11:822–829.
67. Dave BR. Integrated operation theater spine suite (IOTSS) history of spine surgery: Lord Krishna the first eternal spine surgeon. Back Bone: Spine J 2021-2022;2:56–59.
68. Gebhard FT, Kraus MD, Schneider E, Liener UC, Kinzl L, Arand M. Does computer-assisted spine surgery reduce intraoperative radiation doses. Spine (Phila Pa 1976) 2006 31:2024–2027. discussion 2028.
69. Rasouli JJ, Shao J, Neifert S, Gibbs WN, Habboub G, Steinmetz MP, et al. Artificial intelligence and robotics in spine surgery. Global Spine J 2021;11:556–564.
70. Joseph JR, Smith BW, Liu X, Park P. Current applications of robotics in spine surgery: a systematic review of the literature. Neurosurg Focus 2017;42:E2.
71. Ghasem A, Sharma A, Greif DN, Alam M, Maaieh MA. The arrival of robotics in spine surgery: a review of the literature. Spine (Phila Pa 1976) 2018;43:1670–1677.
72. Staub BN, Sadrameli SS. The use of robotics in minimally invasive spine surgery. J Spine Surg 2019;5:S31–S40.
73. Mao JZ, Agyei JO, Khan A, Hess RM, Jowdy PK, Mullin JP, et al. Technologic evolution of navigation and robotics in spine surgery: a historical perspective. World Neurosurg 2021;145:159–167.
74. Woodard EJ, Leon SP, Moriarty TM, Quinones A, Zamani AA, Jolesz FA. Initial experience with intraoperative magnetic resonance imaging in spine surgery. Spine 2001;26:410–417.
75. Narain AS, Hijji FY, Yom KH, Kudaravalli KT, Haws BE, Singh K. Radiation exposure and reduction in the operating room: perspectives and future directions in spine surgery. World J Orthop 2017;8:524–530.
76. Choi G, Modi HN, Prada N, Ahn TJ, Myung SH, Gang MS, et al. Clinical results of XMR-assisted percutaneous transforaminal endoscopic lumbar discectomy. J Orthop Surg Res 2013;8:14.
77. Pae CU. Why systematic review rather than narrative review. Psychiatry Investig 2015;12:417–419.
78. Zhang B, Liu S, Liu J, Yu B, Guo W, Li Y, et al. Transforaminal endoscopic discectomy versus conventional microdiscectomy for lumbar discherniation: a systematic review and meta-analysis. J Orthop Surg Res 2018;13:169.
79. Phan K, Xu J, Schultz K, Alvi MA, Lu VM, Kerezoudis P, et al. Full-endoscopic versus micro-endoscopic and open discectomy: a systematic review and meta-analysis of outcomes and complications. Clin Neurol Neurosurg 2017;154:1–12.
80. Kamper SJ, Ostelo RW, Rubinstein SM, Nellensteijn JM, Peul WC, Arts MP, et al. Minimally invasive surgery for lumbar disc herniation: a systematic review and meta-analysis. Eur Spine J 2014;23:1021–1043.
81. Lewandrowski KU, Soriano-Sánchez JA, Zhang X, Ramírez León JF, Soriano Solis S, Rugeles Ortíz JG, et al. Regional variations in acceptance, and utilization of minimally invasive spinal surgery techniques among spine surgeons: results of a global survey. J Spine Surg 2020;6:S260–S274.
82. Bhanot A, Raiturker PP, Kashyap A, Arora M. Transforaminal endoscopic surgery in lumbar spine: technical aspects, current status, and evolving scope. Indian Spine J 2020;3:54–65.
83. Komp M, Hahn P, Ozdemir S, Merk H, Kasch R, Godolias G, et al. Operation of lumbar zygoapophyseal joint cysts using a full-endoscopic interlaminar and transforaminal approach: prospective 2-year results of 74 patients. Surg Innov 2014;21:605–614.
84. Kashlan ON, Kim HS, Khalsa SS, Singh R, Yong Z, Oh SW, et al. Percutaneous endoscopic Transforaminal approach for far lateral lumbar discectomy: 2-dimensional operative video. Oper Neurosurg (Hagerstown) 2020;18:E8.
85. Li ZZ, Hou SX, Shang WL, Cao Z, Zhao HL. Percutaneous lumbar foraminoplasty and percutaneous endoscopic lumbar decompression for lateral recess stenosis through transforaminal approach: technique notes and 2 years follow-up. Clin Neurol Neurosurg 2016;143:90–94.
86. Li P, Yang F, Chen Y, Song Y. Percutaneous transforaminal endoscopic discectomy for different types of lumbar disc herniation: a retrospective study. J Int Med Res 2021;49:03000605211055045.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||