AbstractObjectiveDestandau’s endospine technique was initially described for lumbar disc herniation and was later applied for lumbar spinal stenosis. Favorable outcomes have been reported with this technique for lumbar degenerative pathology. This article attempts to review the literature and define the scope of Destandau’s technique in cervical and thoracic pathologies.
MethodsA literature search for the keywords “Destandau” and “endospine” was performed in the PubMed, Cochrane, Scopus, Embase, and MEDLINE databases. The review was conducted according to the Scale for the Assessment of Narrative Review Articles (SANRA) tool.
ResultsIn total, 91 studies were found, out of which three studies employed Destandau’s endospine technique for cervical and thoracic pathologies. Three book chapters describing the Destandau technique in cervical pathology and intradural tumor excision were also included in the review. The technique has been successfully employed by various authors for an anterior or posterior cervical approach to disc herniation, cord decompression, and excision of intradural extra-medullary lesions of the spinal canal. No studies mentioned using the Destandau technique for thoracic disc herniation, traumatic fractures, or ossified ligamentum flavum decompression.
ConclusionDestandau’s endoscopic technique has been applied successfully in anterior and posterior cervical approaches for cervical disc herniation, myelopathy and intradural tumors, and its advantages include less pain, minimal muscle damage, shorter hospital stays, and the preservation of spinal stability/segment mobility. Further studies comparing various techniques would help choose the most patient-friendly technique for specific pathologies.
INTRODUCTIONDr Jean Destandau, MD, neurosurgeon from Bordeaux, France, developed a technique of endoscopic spine surgery in 1993 [1-3]. The Destandau’s technique of mobile endospine tube has been largely employed in lumbar disc herniation and lumbar spinal stenosis with favorable outcomes [4-7]. The technique is based on the principle of laparoscopic triangulation between an endoscope and suction with working instrument. Various approaches to the cervical and thoracic spine ranging from open, microscopic tubular, percutaneous, full endoscopic, biportal endoscopy techniques are available. This article attempts to review and define the scope of Destandau’s technique for cervical and thoracic pathology.
MATERIALS AND METHODSA literature search with keywords “Destandau”, “Endospine” was performed in PubMed, Cochrane, Scopus, Embase, MEDLINE databases (Figure 1). The quality of narrative review was performed using Scale for the Assessment of Narrative Review Articles (SANRA) tool [8].
Destandau’s Technique – Surgical MethodThe system consists of an elliptical outer tube which is docked at the site of pathology and an inner tube/working insert which has four in-built channels for the surgeon to work – one for the scope, one working channel for instruments, one for suction and last one for nerve root retractor. The inner tube/working insert fits into the outer tube with a tightening screw or a rachet-type lock. There is an inherent telescoping movement in between these tubes. The system is compatible with a zero-degree, 18 cm long rigid endoscope. The tube can be angled and rotated in all directions to provide mobility. The channels for endoscope and suction are at an angle of 12 degrees to the working channel for instrument. Because of this angle, one can use 0-degree endoscope as an angled endoscope. This helps in minimizing the fogging of endoscope tip.
Once the outer tube is pushed in between the muscles laterally and the spinous process medially, and if the muscles are not separated off the midline carefully, then muscles will intrude inside the outer tube. The surgeon uses two gauze pieces to retract the muscles laterally. The cranial gauze piece is pushed over the cranial lamina and the caudal gauze piece is pushed over the caudal lamina. The gauze pieces must be attached with a thread so that one will not forget the gauze piece by mistake. Once the soft tissues are cleared over the interlaminar area after docking the outer tube with the help of disc forceps or bipolar coagulation, the inner tube is docked onto the outer tube and endoscopic procedure is started. The suction is used with the left hand and working instrument is in the right hand. With suction in the left hand, the surgeon can move the whole system in medial, lateral, cranial, and caudal direction. The same movements are possible with instrument in the right hand (Figure 2).
RESULTSA total of 91 studies were found, out of which 3 studies [9-11] were found to have employed the Destandau endospine in cervical and thoracic pathology. Three book chapters on Destandau technique in cervical pathology and intradural tumor excision were also included in the review [12-14]. All the studies included were limited to case report or case series (level 4 evidence).
DISCUSSION1. Destandau’s Technique in Cervical SpineThe anterior approach to the cervical spine for discectomy was described in 1955 by Robinson and Smith [15]. Currently, anterior cervical discectomy and fusion is regarded as the gold standard surgical option for cervical disc herniation. However, this technique can be accompanied by considerable approach [16-19] and fusion-related complications [20-22]. Mostofi and Khouzani [9] described anterior cervical foraminotomy by Destandau technique (Figure 3) to limit surgical trauma, avoid fusion, preserve motion segment and enhance post-operative recovery. More than 400 patients underwent surgery using this technique in the Endoscopy Center of Spine Surgery in Bordeaux from 2002 to 2014. They felt the visual field in anterior cervical foraminotomy by Destandau technique is broad and depending on the workability of endospine an adequate access even to two cervical levels is possible. The nerve root is decompressed under direct vision and unlike conventional anterior decompression under the microscope, the surgeon does not need to stop and change the position of the surgical operating microscope. There is no need to maintain retraction on esophagus and carotids when the endospine tube is placed hence the risk of esophagus and carotid artery injury can be minimized.
Taran et al. [10] reported using Destandau technique to perform a transoral intralesional excision of C2 chordoma in a 44-year-old lady with prior C1-4 posterior fixation followed by post-operative radiotherapy. They reported no recurrence, metastasis or significant neural deficit at 3 years follow-up.
Endospine can be used for anterior cervical foraminotomy and partial vertebrectomy as described by Jho [23,24]. Rohidas [12] used Jho’s Technique (Figure 4) through Endospine for anterior cervical foraminotomy, discectomy and cord decompression in 55 patients from 2006 to 2016 with excellent results in fifty-two, good in two, fair result in one patient. Dural puncture occurred in one patient which was sealed with muscle piece and fibrin glue; two patients developed Horner’s syndrome; two patients developed transient recurrent laryngeal nerve palsy that recovered completely in 2–8 weeks period.
Rohidas and Destandau [13] also described Destandau technique (Figure 5) for posterior cervical laminooraminotomy and posterior endoscopic cervical canal decompression via unilateral approach in 30 patients from 2004 to 2016. He reports excellent results in 25 patients, 4 good and 1 fair result as per Macnab’s criteria. One patient had a dural tear which was sealed with small muscle and fibrin glue. The author claims an advantage of less hospital stays, less post-operative pain with preservation of motion segment with equivalent efficacy in comparison to open procedures. They advised against posterior approach in cervical instability with deformity, symptomatic central disc hernia with myeloradiculopathy and diffuse ossification of posterior longitudinal ligament causing anterior cord compression.
2. Destandau’s Technique in Thoracic SpineThe interlaminar approach for Thoracic Disc Herniation has been abandoned because of the high incidence of cord damage associated with the procedure [25]. Additionally, in the thoracic region, the window is very small or absent, meaning that more bone removal is required for entry into the canal. The sole indication for an interlaminar approach would be a migrated disc lying dorsal to the spinal cord [25].
We could not find any literature on Destandau Technique for decompression of thoracic ossified ligamentum flavum and traumatic dorsal fractures.
3. Destandau’s Technique in Intradural Extramedullary Spinal LesionPosterior midline laminectomy has been successfully applied as the standard microsurgical technique for the treatment of spinal intradural pathologies. Minimally invasive approaches for intradural tumors have been found to be safe and effective [26-28]. Unilateral hemilaminectomy for intradural tumors using endoscopic assistance has been used successfully for intradural spinal tumors with preservation of musculoligamentous attachments and posterior bony elements [29]. Parihar et al. [11] reported their experience of 18 cases using Destandau’s technique (Figure 6). They included lesions extending up to two vertebral segments. There were 13 schwannomas and 5 meningiomas. All patients improved to normal neurologic functions (modified Frankel grade E) after surgery at follow-up except one patient who was wheelchair bound preoperatively, who also improved and became ambulatory and could walk independently without any support with normal bladder and bowel functions (modified Frankel grade D 3c). Rohidas and Destandau [14] described Destandau’s technique (Figure 7) for intradural extramedullary spinal lesions in 17 cases from 2004 to 2016. Fourteen patients had neurofibroma at lumbar and thoracic level and three cases had meningioma. Sixteen patients recovered completely and one patient had partial recovery of spastic paraplegia. Closure of dura was achieved with 2 mm titanium anastoclips. No CSF leak or wound infection were noted in all patients.
Although endoscopic approaches have many advantages, they are also associated with some limitations such as difficulties in tumor localization, removal of a large tumor, primary dural suturing, control of bleeding, a steep learning curve, and difficulties in bimanual dissection. Improving endoscopic skill using bimanual dissection, hemostasis, and suturing can be learned by attending live operative workshops, cadaveric dissection, watching operative videos, visiting other departments, and watching skillful neuroendoscopic surgeons [30].
4. Destandau Technique Advantages, Limitations and ComplicationsThe endospine technique can be applied at all spinal levels of degenerative spinal pathology – via anterior cervical, posterior cervical, posterior thoracic approach. It can be used in symptomatic disc herniations, spinal stenosis, intradural tumor excision/dural repair. It has also been utilized in debulking of vertebral body tumor (axial chordoma) as reported by Taran et al. [10]. Being a mobile operating tube, it can be tilted and rotated in any direction to address more than one level through a single skin incision. A fixed microscopic system requires multiple tubes of various diameters and lengths depending on patient body fat, build and type of pathology to be addressed; leading to increase in operative inventory which is minimized by use of single endospine tube. This can be advantageous for setups in less developed areas and for travelling surgeons who can minimize the overall cost. Once the surgeon can overcome the learning curve, the contralateral over the top decompression can be performed under better visual control as compared to microscopic techniques.
Destandau technique also has limitations as it utilizes a relatively larger skin incision as compared to full-endoscopic and biportal endoscopic techniques. The subperiosteal stripping of muscles done in order to dock the tube during the interlaminar approach can lead to more atrophic changes in the para-spinal muscles when compared to full endoscopic and biportal techniques. Repeated blood soiling of scope tip can be troublesome when the scope is advanced deeper into the field- this is not an issue in saline medium endoscopy. The system is only compatible with a zero-degree rigid endoscope whereas other endoscopic systems can employ zero degree, 15 degrees, 30 degrees endoscope to allow improved field of view. The system cannot be employed for performing lumbar fusions. As with any technique, there is a learning curve to be overcome initially to get proficient with the technique.
Complications like dural tear, nerve root injury, wrong level surgery, inadequate decompression, recurrence, infection, facet overcutting, instability can occur as with any other open, microscopic and endoscopic techniques.
5. Study LimitationsAll the studies using Destandau’s technique for cervical and thoracic approach were case report and case series by various authors (level 4 evidence). There were no study comparing efficacy of Destandau’s technique with different techniques like open, microscopic approach for cervical and thoracic pathology. The available literature for Destandau’s technique for cervical and thoracic approach is very limited. Further studies focusing on comparing different open, microscopic and endoscopic techniques amongst each other would be helpful in deciding the best technique suited for a particular pathology.
CONCLUSIONThe endoscopic Destandau’s technique has been applied successfully in anterior, posterior cervical approach for cervical disc herniation, myelopathy and intradural tumors with advantage of less pain, minimal muscle damage, less hospital stays and preservation of spinal stability/segment mobility. There is no literature mention of the technique being used for thoracic disc herniation, dorsal traumatic fractures or decompression of ossified ligamentum flavum. Further studies comparing various techniques among each other would help choose the most patient friendly technique for the particular pathology.
NOTESFig. 1.Flowchart of the literature search for the use of Destandau’s technique for cervical and thoracic approaches. 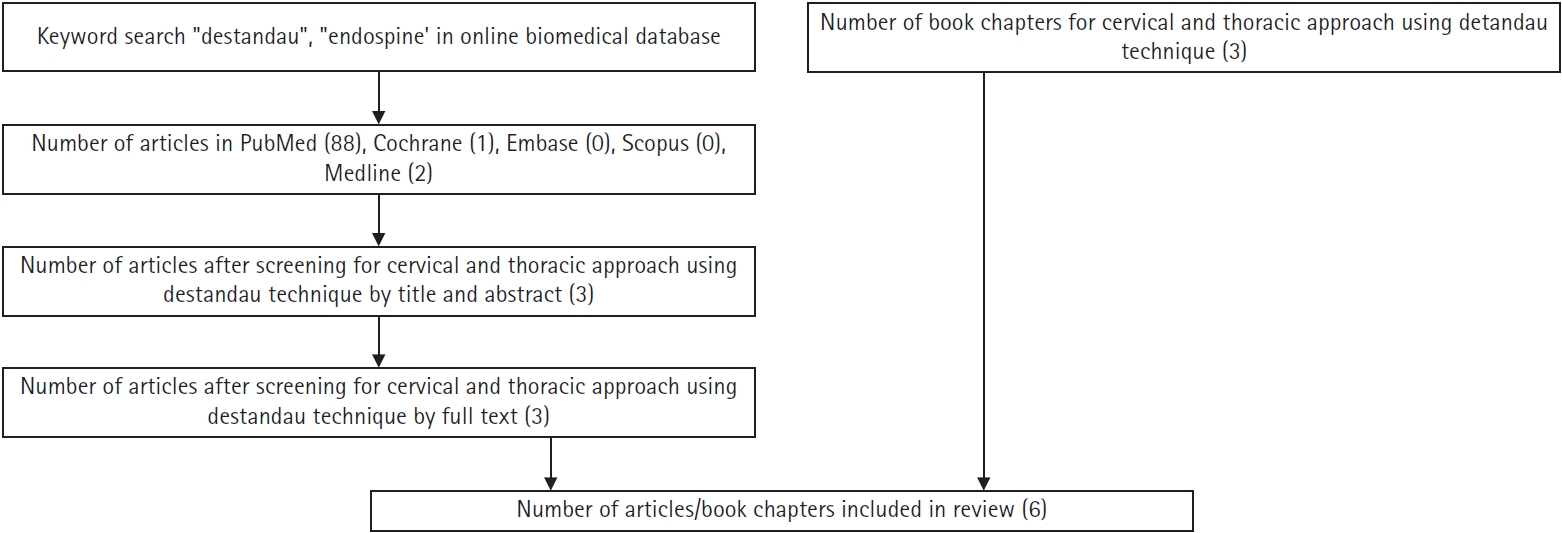
Fig. 2.(A) Inner and outer tubes. (B) Inner tube view of ports from above. (C) Different channels for suction, the endoscope, working instrument, nerve root retractor. (D) Outer tube placed with two gauze pieces and a long thread. (E) Inner tube docked over the outer tube. (F) Outside view of suction in the left hand and instrument in the right hand, controlling the mobility of the tube. 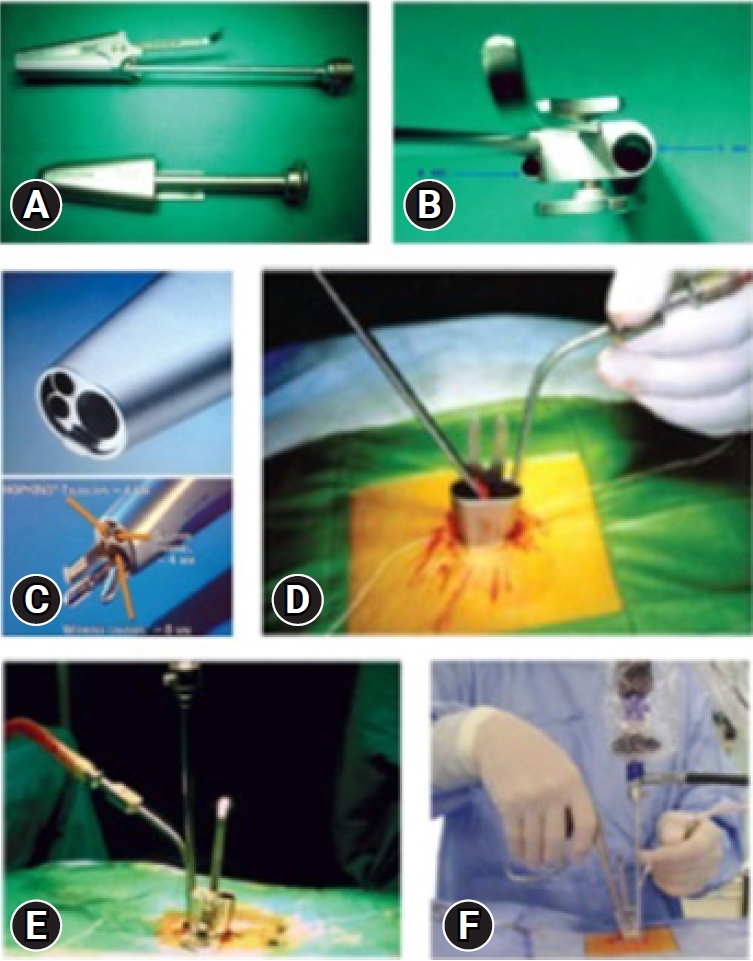
Fig. 3.Cervical localization pin, X-ray level check, and clinical use of the localizing pin in a patient for the anterior approach. 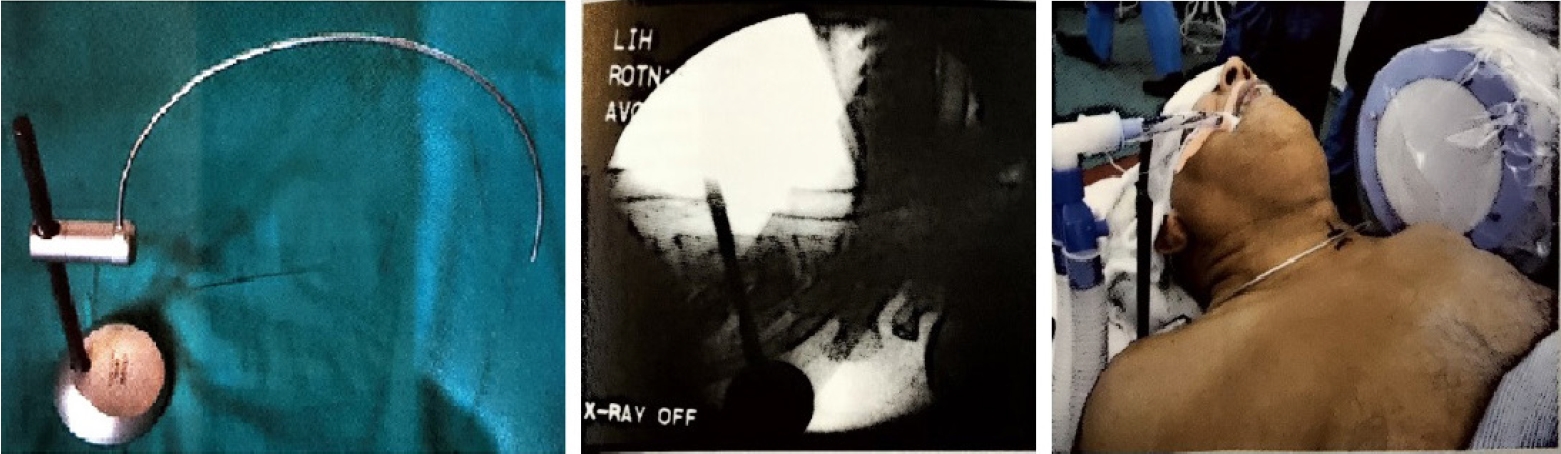
Fig. 4.The anterior Jho approach in a cadaver, anterior endospine approach for cord and root decompression, and clinical scar from the anterior endospine approach. 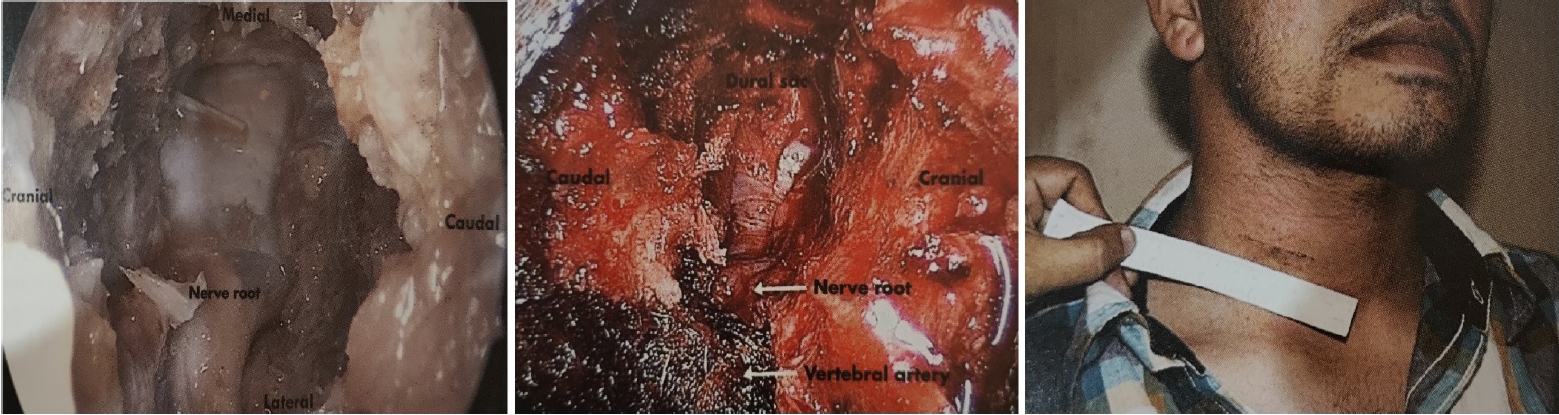
Fig. 5.Patient positioning, using a localizing pin for the posterior approach, posterior lamino-foraminotomy, and posterior cord decompression. 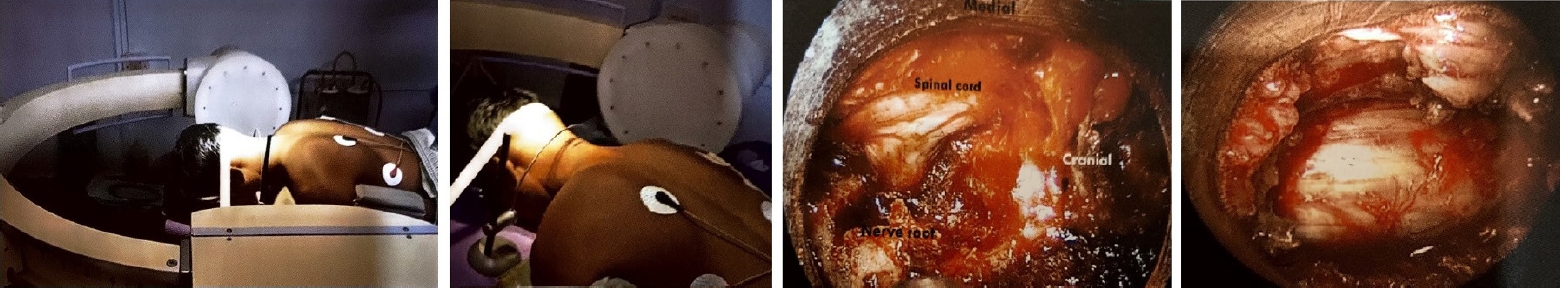
Fig. 6.Endoscopic images showing exposure of the lamina (A), drilling of ipsilateral lamina (B), undercutting of the contralateral lamina (arrow) (C), exposure of the thecal sac after removal of bone and ligamentum flavum (D), dural incision (E), removal of the tumor using the bimanual technique (F, G), direct repair of the dura mater using a fine needle (H), and a knot pusher (I) for dural suturing. 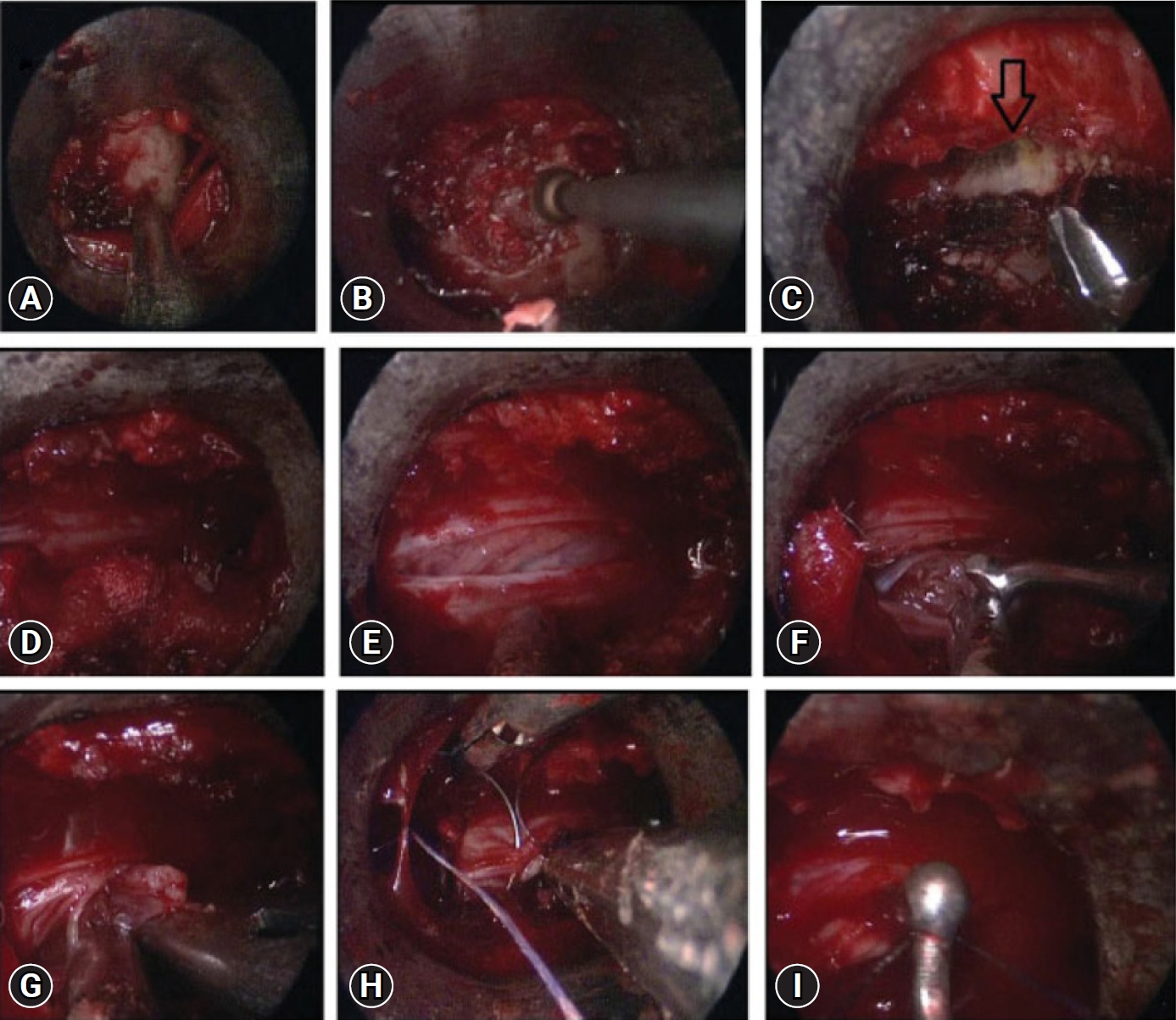
REFERENCES1. Destandau J. A special device for endoscopic surgery of lumbar disc herniation. Neurol Res 1999;21:39–42.
2. Destandau J. Endoscopically Assisted Microdiscectomy. In: Savitz MH, Chiu JC, Yeung AT, editors. The Practice of Minimally Invasive Spinal Technique. Richmond: AAMISMS Education; 2000. p. 187–192.
3. Destandau J. Aspects techniques de la chirurgie endoscopique des hernies discales foraminales lombaires. À propos de 191 case Technical features of endoscopic surgery for lumbar disc herniation: 191 patients. Neurochirurgie 2004 Mar;50(1):6–10. French. doi: 10.1016/s0028-3770(04)98300-2. PMID: 15097915.
4. Kaushal M, Sen R. Posterior endoscopic discectomy: results in 300 patients. Indian J Orthop 2012;46:81–85.
5. Dey PC, Nanda SN. Functional outcome after endoscopic lumbar discectomy by Destandau’s technique: a prospective study of 614 patients. Asian Spine J 2019;13:786–792.
6. Gupta S, Marathe N, Chhabra HS, Destandau J. Long-term functional outcomes of endoscopic decompression with Destandau technique for lumbar canal stenosis. Asian Spine J 2021;15:431–440.
7. Lysoń T, Mariak Z, Jadeszko M, Kochanowicz J, Lewko J. Results of Destandau micro endoscopic lumbar discectomy. Neurol Neurochir Pol 2008;42:105–111.
8. Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev 2019;4:5.
9. Mostofi K, Khouzani RK. Endoscopic anatomy and features of anterior cervical foraminotomy by Destandau technique. Open Access Maced J Med Sci 2016;4:650–653.
10. Taran S, Yusof AH, Yusof MI. Endoscopic transoral resection of an axial chordoma: a case report. Malays Orthop J 2015;9:75–77.
11. Parihar VS, Yadav N, Yadav YR, Ratre S, Bajaj J, Kher Y. Endoscopic management of spinal intradural extramedullary tumors. J Neurol Surg A Cent Eur Neurosurg 2017;78:219–226.
12. Rohidas SM, Destandau J. Chapter 10. Endoscopic Anterior Cervical Discectomy and Cord Decompression. Endoscopic Spine Surgery: Destandau’s Technique. Uttar Pradesh: Thieme; 2017. p. 145.
13. Rohidas SM, Destandau J. Chapter 9. Posterior Cervical Endoscopic Discectomy. Endoscopic Spine Surgery: Destandau’s Technique. Uttar Pradesh: Thieme; 2017. p. 131.
14. Rohidas SM, Destandau J. Chapter 11. Endoscopic Removal of Intradural Extramedullary Space-Occupying Lesion. Endoscopic Spine Surgery: Destandau’s Technique. Uttar Pradesh: Thieme; 2017. p. 173.
15. Robinson RA, Smith GW. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull John Hopkins Hosp 1955;96:223–224.
16. Flynn TB. Neurologic complications of anterior cervical interbody fusion. Spine (Phila Pa 1976) 1982;7:536–539.
17. Bulger RF, Rejowski JE, Beatty RA. Vocal cord paralysis associated with anterior cervical fusion: considerations for prevention and treatment. J Neurosurg 1985;62:657–661.
18. Pedram M, Castagnera L, Carat X, Macouillard G, Vital JM. Pharyngolaryngeal lesions in patients undergoing cervical spine surgery through the anterior approach: contribution of methylprednisolone. Eur Spine J 2003;12:84–90.
19. Wang MC, Chan L, Maiman DJ, Kreuter W, Deyo RA. Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine (Phila Pa 1976) 2007;32:342–347.
20. Maiman DJ, Kumaresan S, Yoganandan N, Pintar FA. Biomechanical effect of anterior cervical spine fusion on adjacent segments. Biomed Mater Eng 1999;9:27–38.
21. Epstein NE. A review of laminoforaminotomy for the management of lateral and foraminal cervical disc herniations or spurs. Surg Neurol 2002;57:226–233.
22. Kulkarni V, Rajshekhar V, Raghuram L. Accelerated spondylotic changes adjacent to the fused segment following central cervical corpectomy: magnetic resonance imaging study evidence. J Neurosurg 2004;100:2–6.
23. Jho HD. Microsurgical anterior cervical foraminotomy for radiculopathy: a new approach to cervical disc herniation. J Neurosurg 1996;84:155–160.
24. Jho HD. Spinal cord decompression via microsurgical anterior foraminotomy for spondylotic cervical myelopathy. Minim Invasive Neurosurg 1997;40:124–129.
25. Börm W, Bäzner U, König RW, Kretschmer T, Antoniadis G, Kandenwein J. Surgical treatment of thoracic disc herniations via tailored posterior approaches. Eur Spine J 2011;20:1684–1690.
26. Gandhi RH, German JW. Minimally invasive approach for the treatment of intradural spinal pathology. Neurosurg Focus 2013;35:E5.
27. Lu DC, Chou D, Mummaneni PV. A comparison of mini-open and open approaches for resection of thoracolumbar intradural spinal tumors. J Neurosurg Spine 2011;14:758–764.
28. Mannion RJ, Nowitzke AM, Efendy J, Wood MJ. Safety and efficacy of intradural extramedullary spinal tumor removal using a minimally invasive approach. Neurosurgery 2011;68:208–216.
|
|
||||||||||||||||||||||||||||||||||||||||||