AbstractObjectiveUnilateral biportal endoscopic (UBE) treatment for lumbar disc herniation (LDH) is an advanced surgical procedure that has recently gained popularity. Numerous reports from developed countries have demonstrated the effectiveness of this minimally invasive technique. We evaluated the initial outcomes of UBE at a healthcare facility with limited resources.
MethodsClinical and radiographic data of 82 patients with LDH treated between July 2022 and June 2023 using UBE discectomy techniques, including the ipsilateral interlaminar approach, contralateral sublaminar approach, and paraspinal approach, were reviewed. Outcomes were analyzed in terms of the modified MacNab criteria, Japanese Orthopaedic Association (JOA) score, and visual analogue scale (VAS), with a mean follow-up of 3.1 months.
ResultsAt the final follow-up, the mean VAS for low back pain improved from 4.5±1.0 to 1.2±0.4 and the VAS for leg pain improved from 7.8±0.9 to 1.6±0.5. The mean JOA score improved from 13.5±2.4 to 24.2±2.1. The modified MacNab criteria were excellent in 56 patients (68.3%), good in 22 (26.9%), and fair in 4 (4.8%). In total, 106 levels of LDH were treated. L4–5 disc herniation was performed in 55 patients (51.9%), L5–S1 in 36 (34.0%), L3–4 in 8 (7.5%), L2–3 in 6 (5.7%), and L1–2 in 1 (0.9%). The ipsilateral interlaminar approach was performed in 93 patients (87.7%), the contralateral sublaminar approach in 7 (6.6%), and the paraspinal approach in 6 (5.7%). Operative time significantly improved after performing 20 cases. In the early stage (1–20 cases), the operation time per level was 102.0±28.2 min, while in the next stage (21–82 cases) it was 78.1±20.4 minutes. No serious complications, including cauda equine syndrome or root palsy, were observed. Three patients had dural tears (2.8%), and 1 had epidural hematoma (0.9%).
INTRODUCTIONLumbar disc herniation (LDH) is a prevalent cause of lower back pain and can lead to sensory or motor disturbances in the lower limbs. This condition occurs when protruding disc material compresses spinal nerve roots. Initially, the treatment for LDH is conservative and includes the use of analgesics, physiotherapy, and epidural steroid injections. However, surgical intervention becomes necessary for patients who do not respond to conservative measures, particularly when neurological deficits are present and supported by radiological findings [1-5]. Over the past few decades, surgical approaches for LDH have evolved from open procedures to minimally invasive surgeries (MISs). MIS techniques, such as microdiscectomy (MD), percutaneous endoscopic lumbar discectomy (PELD), also known as uniportal full endoscopic, and unilateral biportal endoscopic discectomy (UBED), prioritize the preservation of the spine's normal anatomy. These MIS techniques offer several advantages over traditional methods, including reduced damage to paraspinal muscles, preservation of bone structures, minimized blood loss, and quicker recovery times [6]. However, it's important to note that PELD necessitates a steep learning curve and specialized surgical equipment [7-11]. In contrast, UBED is an emerging option that partly addresses these challenges. By employing two separate channels for surgical procedures and visualization, UBED enables surgeons to quickly adapt to the technique and utilize various conventional instruments, such as curettes, Kerrison punches, osteotomes, high-speed drills, and standard forceps which helps mitigate the financial burden associated with specialized equipment [12-17]. In low- and middle-income countries with limited resources for medical training and practice, adopting new surgical techniques can be challenging. However, by participating in short training courses and making use of available equipment like arthroscopy and conventional open spine surgical devices, spine surgeons can achieve promising results with the UBED technique. In this study, we present preliminary outcomes for LDH patients who underwent UBED at a resource-constrained institution.
MATERIALS AND METHODS1. Study Design and ParticipantsA retrospective review was conducted on patients who had undergone UBED by a single surgeon following a diagnosis of LDH between July 2022 and June 2023. This study received approval from from Institutional Review Board of Xuyen A General Hospital Medical Research Council. Because this study reviewed preexisting data, informed consent was waived.
The inclusion criteria were as follows: (1) a clear diagnosis of LDH with significant lower extremity radiating pain, low back pain, and lower extremity motor and/or sensory dysfunction; (2) computed tomography (CT) scan and magnetic resonance imaging (MRI) of the lumbar spine consistent with clinical symptoms and signs; (3) unsuccessful conservative treatment for a minimum of 4 weeks. The exclusion criteria were as follows: (1) lumbar spinal stenosis confirmed by MRI; (2) presence of segmental instability confirmed by dynamic radiographs; (3) lumbar spine infection, tumor, or trauma; (4) history of previous lumbar spine surgery.
2. Surgical ProceduresAll surgical procedures were performed by a single right-handed surgeon. Patients were placed in the prone position under general anesthesia. The surgical level and landmarks are determined during fluoroscopy direction. The target point is identified by using a true-anteroposterior view as the lower part of the cranial lamina. Generally, the working portal is made on the disc level first by a transverse skin incision, and then the scope portal is made 2.5–3.0 cm apart from it. Two skin incisions are usually located along the medial pedicle line (Figure 1). If multilevel are planned, the endoscopic portal can be used in the instrumental portal for the next.
We used the UBE instrument kits from BONSS Medical (BONSS Medical Technology Co., Ltd., Jiangsu, China). Through the viewing portal, a 0° arthroscope was inserted. After that, a saline irrigation pump or a 1,000-mL saline bag placed 50 cm above the patient’s back was connected, which could maintain approximately 30-mmHg pressure during the procedure. The working space, which was identified from the lower border of the cranial lamina to the upper border of the caudal lamina, was prepared by using a serial dilator or muscle detacher. The radiofrequency (RF) coagulator, muscle shaver or pituitary forceps was used to remove the soft tissues to confirm the landmarks. A meticulous hemostasis would be performed continuously to create a clear surgical field. The successful initial working space was formed when two portals’ distal endpoints must meet just on the laminar.
We always start laminectomy by using an arthro-shaver (Ergo 2-Button Shaver Handpiece) or a high-speed drill with a 4-mm diamond burr from the spinous-laminar junction to the upper free margin of the ligamentum flavum (LF) cranially. It extends laterally until encountering the meeting point of inferior articular process and superior articular process. During this step, the LF was left as a protector to avoid neural injury or dural tear until bone working is finished (Figure 2).
Two layers of LF were removed sequentially from cranial to caudal and medial to lateral with Kerrison punch, alligator forceps, pituitary forceps, and curette. After full ipsilateral flavectomy, we would clearly identify the epidural space including fat tissue and dural mater.
We used a retractor to protect nerve root safely before discectomy. Firstly, the ruptured disc fragments were removed easily to partly decompress. Secondly, depending on the location and characteristics of the disc herniation, the subsequent discectomy would be performed carefully. Soft disc materials were taken out with conventional forceps, whereas calcified particles were detached with osteotomes. Finally, related to the risk of recurrence, internal disc decompression was performed using RF coagulator annuloplasty. The sufficient decompression was confirmed by using the blunt hook to expose the perineural space including shoulder and axillar portion nerve root with freely movement (Figure 3). In every UBED procedure, meticulous bleeding control was conducted prior to applying skin closure. A drainage catheter was then carefully inserted over the dura mater through the working portal. This catheter has been removed on the second day post-op during the assessment of the surgical site, ensuring that it remained dry and exhibited no signs of fluid oozing.
3. Observation Indicators(1) General patient demographics and condition: age, sex, body mass index and underlying disease; (2) Preoperative-related indexes: symptoms duration, preoperative visual analogue scale (VAS) score for low back pain and leg pain, Japanese Orthopaedic Association (JOA) score; (3) Indexes related to operation: operative level, approach technique, operative time, amount of bleeding; (4) postoperative-related indicators: postoperative hospital stay, VAS score, JOA score, modified-MacNab criteria, and complications.
RESULTS1. Demographic DataA total of 82 patients who underwent UBED for LDH was recruited in the present study. The average follow-up period was 3.1 months (range, 1–12 months). The patients’ demographic and preoperative characteristics are shown in Table 1.
2. Clinical DataA total of 106 levels of LDH were done (Table 2).
The operation time was 83.9±24.6 minutes (range, 60–150 minutes) per level of UBED. Operative time significantly improved after performing 20 cases.
Intraoperative blood loss was minimal. The postoperative hospital stay was 4.7±2.1 days (range, 2–10 days). Most of the patients got off the bed for ambulation on the 2nd postoperative day. The preoperative characteristics and outcomes are listed in Table 3.
No serious complications, such as cauda equina syndrome or root palsy, were observed. Three patients (2.8%) had small dural tears. Successful repair under endoscopy was performed in one patient by using 6/0 Vicryl sutures, while the remaining two were managed conservatively with a 24-hour period of bed rest and observation. None of the cases resulted in cerebrospinal fluid leakage or necessitated revision surgery due to sustained headache or nerve deficits. An additional complication noted was the occurrence of an epidural hematoma in 1 patient (0.9%), characterized by severe and persistent low back pain postoperation. We addressed this issue through the administration of pain medications and nonsteroidal anti-inflammatory drugs, along with subsequent follow-up MRI. Given the absence of symptoms indicating root compression, reoperation was considered unnecessary, and the back pain showed improvement after 1 month. There was no infection or wound-related complications.
DISCUSSIONUnilateral biportal endoscopic (UBE) was first described in 1996 by De Antoni et al. [18] when performing surgery for LDH, using separate channels for the scope and operating instruments. Subsequently, continuous improvements in surgical techniques and support tools have been made, especially by Korean surgeons. In 2017, Son et al. [19] reported the implementation of UBE technique for treating various spinal pathologies, including spinal canal stenosis and posterior interbody fusion [19]. The term “biportal” officially started being used in 2016 to distinguish it from the uniportal-full endoscopic [20]. As of the present time, numerous studies have shown the effectiveness and safety of this technique in treating lumbar spinal diseases [14,17,19-28]. However, most of these studies were conducted by expert surgeons in well-equipped medical facilities. In 2013, Soliman [27] followed 41 cases of LDH treated with UBED. The results showed significant improvements in VAS scores, Oswestry Disability Index, and a high satisfaction rate of 95% (39 of 41) according to the MacNab criteria. Additionally, the average surgery time was significantly reduced from 93 minutes in the first 10 cases to 60 minutes in the subsequent 31 cases. Kim et al. [29] conducted a comparative study with data from 60 cases of UBED and 81 cases of MD. Clinical improvements based on VAS scores and the rate of good recovery according to the Macnab criteria were similar, with 73.4% in the UBED group and 68.5% in the MD group. Although there was significantly less blood loss and shorter hospital stays in the UBED group, the operation time was longer. In 2019, Lin et al. [30] reported a systematic review, comprising 11 previously published studies involving 556 patients and 679 levels of lumbar spinal diseases, the mean follow-up was 15.2 months, showed an 84.3% excellent/good recovery rate (range, 75.35%–95%) according to the MacNab criteria and the mean length of hospital stay was 4.4 days. There were similar results between UBED for the treatment of LDH and stenosis. Our study observed similar results. The effectiveness of clinical improvement is clearly evident through changes in VAS scores for low-back pain, VAS scores for leg pain, and JOA scores (Table 3). Paired-sample t-tests comparing the 2 postoperative time points (discharge and the last follow-up) with the preoperative values all showed statistically significant differences. The majority of patients achieved a good recovery outcome (68.3%) based on MacNab criteria. The average postoperative hospital stay was 4.7 days (range, 2–10 days). All patients were able to start walking out of bed on the second postoperative day.
Several advantages of UBED are contributing to its gradual replacement of conventional open surgery and MD, as it continues to evolve alongside PELD [29]. The primary advantage, acknowledged by all authors, is that UBED is a MIS technique that maximizes the preservation of the anatomical structures of the spine, thereby promoting rapid patient recovery [6,16,30,31]. Recently, a study conducted by So and Park [32] investigated differences in bone healing at the postoperative laminectomy site between MD and UBED for the treatment of LDH, with a 6-month follow-up. The conclusion indicated that, in comparison to the MD group, the UBED group exhibited a larger area of bone healing and a higher bone recovery ratio in patients undergoing lumbar discectomy. These findings suggest that preserving normal structures is more achievable during UBED than during MD. One of the key aspects of the UBED technique is accessing and creating an operative space with minimal damage to the multifidus muscles whenever possible. In addition to preservation of paraspinal muscles, the protection of multifidus muscles is particularly emphasized by Son to realize the true benefits of the UBED concept [33].
Other advantages can make UBED feasible in surgical centers that may have certain limitations in terms of resources. This primarily stems from the surgical techniques, learning curve and investment in instruments. Firstly, with the principle of 2 independent portals, 1 for the vision (scope) and 1 for the operation (working), it allows for the expansion of instrument movement within the surgical field while maintaining clear observation. By utilizing the flexibility of the scope's viewing, surgeons find it easier to access positions that might have been challenging in microscopic, which is hindered by dependence on the direction of light through the tubular retractor. Furthermore, UBED primarily utilizes conventional surgical instruments such as Kerrison punches, various types of forceps, and curettes, which partly contribute to reducing the time required for their use [12,13,15,34]. In this study, we observed a significant difference in operative time per level between the 2 groups: group 1 consisting of the first 20 cases and group 2 consisting of the subsequent 62 cases. The operation time showed a downward trend as a whole and the scatter chart of the operation time is shown in Figure 4. It should be noted that there was no significant difference in preoperatvie indicators, including basic diseases, duration of symptoms, and preoperative JOA score. Additionally, the age of patients in group 1 is younger than that in group 2 (Table 4). The improvement of operative time was attributed to the streamlined coordination of anesthesia, surgical field preparation, water system management, proficiency in using instruments. This observation aligns with findings from several previous studies. In 2022, a study conducted by Chen et al. [12] utilized the CUSUM (Cumulative Sum) method to delineate the learning curve for performing UBED in single-level LDH, establishing a cutoff point at 24 cases. The authors also referenced multiple reports and compared their findings with those of PELD, which had cutoff points ranging from 40 to 70 cases depending on the specific study [7,8]. The UBED procedure was conducted at our institution after we completed a brief Cadaveric Surgical Training course (lasting 3 days in Thailand) and attended several continuing medical education (CME) courses. These courses included a 2-week program at the International UBE Academy in Busan, Korea and 2 CME courses at the University of Medicine and Pharmacy in Ho Chi Minh City, Vietnam.
Moreover, the use of mostly open conventional surgical instruments also helps reduce the equipment investment costs. Currently, in Vietnam, there is only one company providing UBE equipment from BONSS (BONSS Medical Technology Co., Ltd.). In 2020, Wu [34] proposed an octagonal model consisting of 8 factors to consider when implementing endoscopic spine surgery, including the aspect of equipment provision. This report indicates that the cost for PELD was higher compared to UBED. PELD necessitates specialized endoscopes and dedicated instruments tailored to each approach, while several instruments used in UBED can be repurposed from those already utilized and readily available in a spine operative unit. Examples include arthro-scope, open spine instruments, RF energy systems, and water pressure systems commonly used in arthroscopic surgery. In fact, during the initial phase of implementing the UBED procedure, we performed laminectomy using the arthro-shaver (Ergo 2-Button Shaver Handpiece) with a 4-mm diamond-coated burr at a maximum speed of 7,000 RPM, instead of using high-speed drills for neurosurgery in later stages. To address the absence of the complete water pressure control system, we used the saline bags and adjusted them by estimating the height relative to the surgical field, similar to the method described by Son [33]. In terms of cost-effectiveness, a study conducted by Choi et al. [15] compared four lumbar discectomy techniques, including MD and 3 endoscopic discectomy (ED) procedures. The authors observed that both the direct and indirect costs of MD were significantly higher than those associated with three ED methods. Additionally, there was no significant difference in cost-effectiveness between UBED and other ED techniques. In our study, we did not have data to compare treatment costs with other methods. However, National Health Insurance partially supports the costs, and the primary hospital costs (the sum of the costs associated with the operation, surgical equipment such as disposable RF probes, hemostatic agents, antiadhesive agents, anesthesia, hospital stay including meals, nursing care, laboratory work, postoperative intravenous patient-controlled analgesia, physical therapy and/or medication, and radiological examinations including CT and MRI) at our hospital are equal for PELD and UBED.
Regarding the surgical procedure, our primary method of accessing the lesions was through the ipsilateral interlaminar approach, located adjacent to the site of the disc herniation (87.7% of cases). This surgical approach offers the advantage of familiar anatomical landmarks and easy identification [17,35]. The contralateral sublaminar and paraspinal approaches were reserved for foraminal lesions, which required a more experienced surgeon. In the context of addressing high-grade migrated disc herniations, the UBED with an interlaminar approach is preferred choice [36]. Additionally, the interlaminar approach in endoscopic procedures has gained popularity over the transforaminal when dealing with disc herniations at the L5–S1 level. The transforaminal approach to L5–S1 encounters limitations due to factors such as a prominent iliac crest, hypertrophy of the L5–S1 facet joint, and the inherently narrower L5–S1 foramen [37-39].
The safety of UBED is evident in the low incidence of perioperative complications. In 2022, a systematic review of 22 studies with various lumbar spine pathologies and UBE techniques, conducted by Li et. [31], revealed that the complication rate predominantly remained below 11%. Among these studies, 16 specifically focused on a total of 596 LDH patients who underwent UBED, resulting in an overall perioperative complication rate of 5.37%. Notably, Kang et al. [17] reported on 262 patients with LDH, including 54 cases of high-grade migrated herniations (zone 1 and zone 4), and no complications were reported in any of these cases. In a recent systematic review and meta-analysis conducted by He et al. [16], 7 studies were included that reported on the occurrence of perioperative complications. The combined findings from these studies revealed that there was no statistically significant difference in the incidence of perioperative complications between patients undergoing UBED and those undergoing PELD. What's noteworthy is that when the results of four studies were pooled together, it was observed that UBED appeared to be associated with a lower rate of LDH recurrence during the follow-up period in comparison to PELD, particularly among patients with single-level LDH.
In summary, the initial findings from the implementation of UBED, combined with a retrospective literature review, suggest that UBED is an effective, safe, and feasible technique for managing patients with LDH in limited resources hospitals. Nevertheless, several limitations should be acknowledged within this study. Firstly, since it is a retrospective review, potential biases may have influenced the data collection process. Additionally, this study is conducted in a single-center setting, with all procedures performed by the same surgeon who is experienced in both microscopic and uniportal full endoscopic surgeries. This homogeneity in surgical expertise could impact operative times and the learning curve for other surgeons adopting the technique. It is important to note that endoscopic spine surgery, in general, presents unique challenges as surgeons must adapt to a 2-dimensional perspective and different spatial handling compared to open surgery. Another noteworthy limitation is the absence of a comparison of primary hospital costs, as they have all been covered by the National Health Insurance. This stems from our focus on the feasibility of UBED with the current hospital equipments and reduced training time, and its potential applicability to similar healthcare institutions. Furthermore, comprehensive postoperative imaging data, especially MRI, were not consistently collected for thorough comparisons. Lastly, the relatively short follow-up period limits our ability to assess long-term complications, particularly the recurrence rate.
CONCLUSIONUBED is a safe and effective technique for the treatment of LDH, demonstrating complete feasibility for implementation in hospitals with limited resources. Following a short training program and utilizing existing equipments, spine surgeons can apply the UBE technique in their practices, leading to positive outcomes. However, further large-cohort prospective and randomized trials, with long-term follow-up, should be performed to evaluate the relative benefits and drawbacks of UBE surgery.
Fig. 1.(A) True anteroposterior view. (B) Skin incision and creation of 2 portals: pedicle (white oval), working portal (green line), and scope portal (red line); the initial targeted area is the spinolaminar junction (yellow circle). (C) Lateral view to double-check the operative level. (D) Surgical field: an arthroscope with continuous saline flow and high-speed drills was inserted. 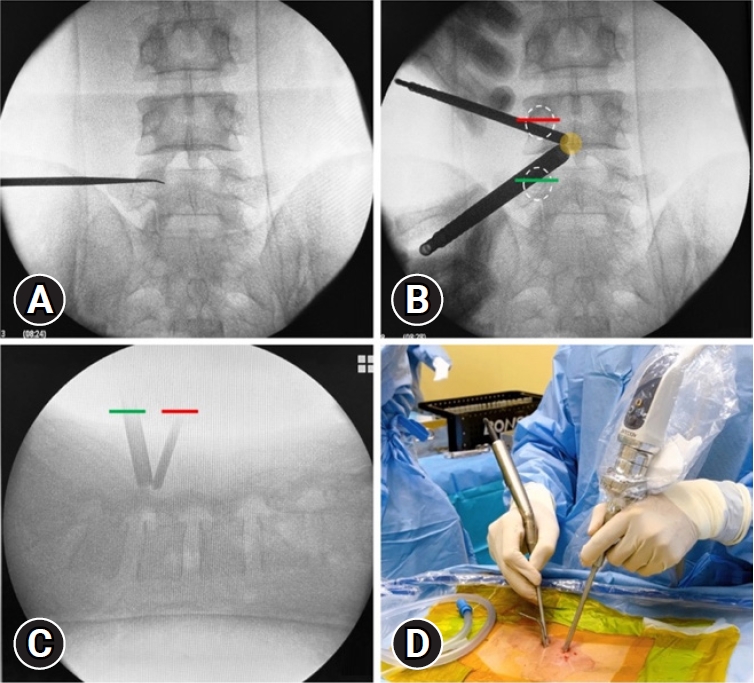
Fig. 2.(A) Laminectomy was started from the spinous-laminar junction, and the lower part of the cranial lamina was identified (dotted white curve). (B) The ligamentum flavum (LF) was left as a protector to avoid neural injury and reduce blood bleeding until bone working was finished. (C) Sufficient laminectomy viewing the upper free margin of the LF (black star). (D) Lateral extension was performed until encountering the point where the inferior articular process and superior articular process met (black arrows), apart from laminectomy of the caudal lamina if required (white star). 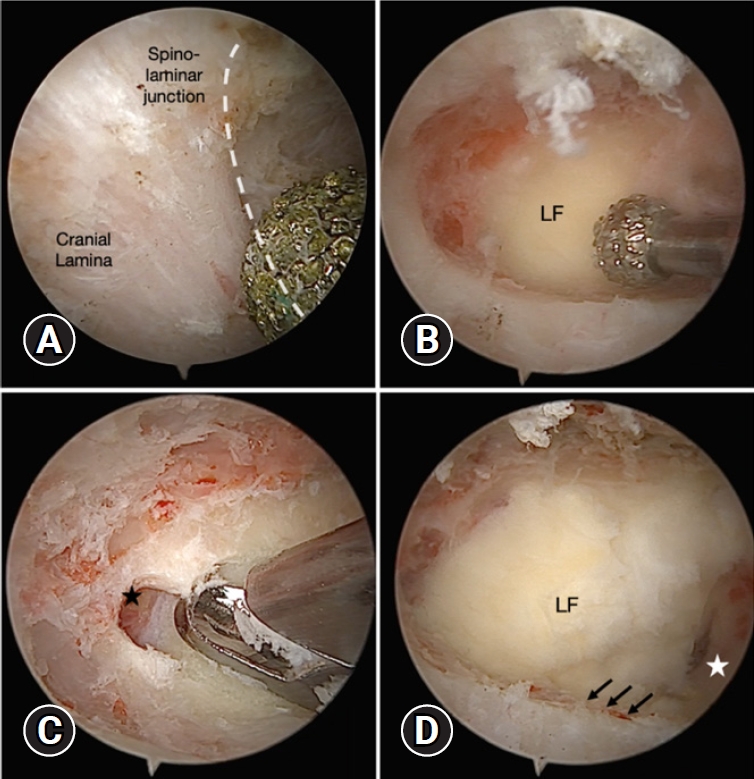
Fig. 3.(A) The disc space (black star) was identified using a retractor (white star) to protect the traversing nerve root (TNR) safely before discectomy. (B) Ruptured fragments and soft disc materials were taken out with conventional forceps. (C) Radiofrequency (RF) coagulator annuloplasty was performed. (D) Sufficient decompression was confirmed by using the blunt hook to expose the perineural space, including the shoulder and axillar portion of the nerve root with free movement. LF, ligamentum flavum. 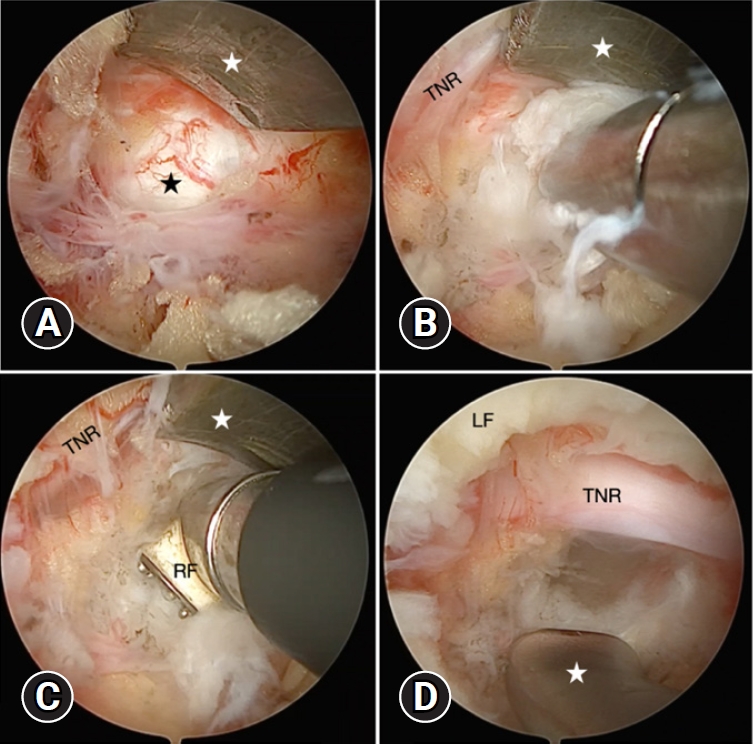
Table 1.Demographic data of 82 patients with LDH in this study Values are presented as mean±standard deviation (range) or number (%). LDH, lumbar disc herniation; BMI, body mass index. †Age-related health disorders in the modified 5-item frailty index: diabetes mellitus, hypertension, chronic obstructive pulmonary disease or recent pneumonia, congestive heart failure, history of cerebral infarction. Table 2.Indexes related to surgery (n=106) Table 3.Clinical data of 82 patients with lumbar disc herniation in this study Table 4.Characteristics of the first 20 cases and the next 62 cases Values are presented as mean±standard deviation. BMI, body mass index; JOA, Japanese Orthopaedic Association. *p<0.05, statistically significant differences. †Paired-sample t-test. ‡Age-related health disorders in the modified 5-item frailty index: diabetes mellitus, hypertension, chronic obstructive pulmonary disease or recent pneumonia, congestive heart failure, history of cerebral infarction. REFERENCES2. Basic Research and Transformation Society, Professional Committee of Spine and Spinal Cord, Chinese Association of Rehabilitation Medicine. [Guideline for diagnosis, treatment and rehabilitation of lumbar disc herniation]. Zhonghua Wai Ke Za Zhi 2022 60:401–8. (Chinese).
3. Benson RT, Tavares SP, Robertson SC, Sharp R, Marshall RW. Conservatively treated massive prolapsed discs: a 7-year follow-up. Ann R Coll Surg Engl 2010;92:147–53.
4. Benzakour T, Igoumenou V, Mavrogenis AF, Benzakour A. Current concepts for lumbar disc herniation. Int Orthop 2019;43:841–51.
5. Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J 2014;14:180–91.
6. Tang K, Goldman S, Avrumova F, Lebl DR. Background, techniques, applications, current trends, and future directions of minimally invasive endoscopic spine surgery: a review of literature. World J Orthop 2023;14:197–206.
7. Morgenstern R, Morgenstern C, Yeung AT. The learning curve in foraminal endoscopic discectomy: experience needed to achieve a 90% success rate. SAS J 2007;1:100–7.
8. Tenenbaum S, Arzi H, Herman A, Friedlander A, Levinkopf M, Arnold PM, et al. Percutaneous posterolateral transforaminal endoscopic discectomy: clinical outcome, complications, and learning curve evaluation. Surg Technol Int 2011;21:278–83.
9. Son S, Ahn Y, Lee SG, Kim WK, Yoo BR, Jung JM, et al. Learning curve of percutaneous endoscopic transforaminal lumbar discectomy by a single surgeon. Medicine (Baltimore) 2021;100:e24346.
10. Yang J, Guo C, Kong Q, Zhang B, Wang Y, Zhang L, et al. Learning curve and clinical outcomes of percutaneous endoscopic transforaminal decompression for lumbar spinal stenosis. Int Orthop 2020;44:309–17.
11. Hsu HT, Chang SJ, Yang SS, Chai CL. Learning curve of full-endoscopic lumbar discectomy. Eur Spine J 2013;22:727–33.
12. Chen L, Zhu B, Zhong HZ, Wang YG, Sun YS, Wang QF, et al. The learning curve of unilateral biportal endoscopic (UBE) spinal surgery by CUSUM analysis. Front Surg 2022;9:873691.
13. Choi DJ, Choi CM, Jung JT, Lee SJ, Kim YS. Learning curve associated with complications in biportal endoscopic spinal surgery: challenges and strategies. Asian Spine J 2016;10:624–9.
14. Choi DJ, Jung JT, Lee SJ, Kim YS, Jang HJ, Yoo B. Biportal endoscopic spinal surgery for recurrent lumbar disc herniations. Clin Orthop Surg 2016;8:325–9.
15. Choi KC, Shim HK, Kim JS, Cha KH, Lee DC, Kim ER, et al. Cost-effectiveness of microdiscectomy versus endoscopic discectomy for lumbar disc herniation. Spine J 2019;19:1162–9.
16. He D, Cheng X, Zheng S, Deng J, Cao J, Wu T, et al. Unilateral biportal endoscopic discectomy versus percutaneous endoscopic lumbar discectomy for lumbar disc herniation: a systematic review and meta-analysis. World Neurosurg 2023;173:e509–20.
17. Kang T, Park SY, Park GW, Lee SH, Park JH, Suh SW. Biportal endoscopic discectomy for high-grade migrated lumbar disc herniation. J Neurosurg Spine 2020 May 15:1–6. doi: 10.3171/2020.2.SPINE191452. [Epub].
18. De Antoni DJ, Claro ML, Poehling GG, Hughes SS. Translaminar lumbar epidural endoscopy: anatomy, technique, and indications. Arthroscopy 1996;12:330–4.
19. Heo DH, Son SK, Eum JH, Park CK. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: technical note and preliminary clinical results. Neurosurg Focus 2017;43:E8.
20. Hwa Eum J, Hwa Heo D, Son SK, Park CK. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine 2016;24:602–7.
21. Park JH, Jang JW, Park WM, Park CW. Contralateral keyhole biportal endoscopic surgery for ruptured lumbar herniated disc: a technical feasibility and early clinical outcomes. Neurospine 2020;17(Suppl 1):S110–9.
22. Park MK, Park SA, Son SK, Park WW, Choi SH. Clinical and radiological outcomes of unilateral biportal endoscopic lumbar interbody fusion (ULIF) compared with conventional posterior lumbar interbody fusion (PLIF): 1-year follow-up. Neurosurg Rev 2019;42:753–61.
23. Eun SS, Eum JH, Lee SH, Sabal LA. Biportal Endoscopic lumbar decompression for lumbar disk herniation and spinal canal stenosis: a technical note. J Neurol Surg A Cent Eur Neurosurg 2017;78:390–6.
24. Heo DH, Lee N, Park CW, Kim HS, Chung HJ. Endoscopic unilateral laminotomy with bilateral discectomy using biportal endoscopic approach: technical report and preliminary clinical results. World Neurosurg 2020;137:31–7.
25. Kim JE, Choi DJ. Unilateral biportal endoscopic decompression by 30° endoscopy in lumbar spinal stenosis: Technical note and preliminary report. J Orthop 2018;15:366–71.
26. Pao JL, Lin SM, Chen WC, Chang CH. Unilateral biportal endoscopic decompression for degenerative lumbar canal stenosis. J Spine Surg 2020;6:438–46.
27. Soliman HM. Irrigation endoscopic discectomy: a novel percutaneous approach for lumbar disc prolapse. Eur Spine J 2013;22:1037–44.
28. Akbary K, Kim JS, Park CW, Jun SG, Hwang JH. Biportal endoscopic decompression of exiting and traversing nerve roots through a single interlaminar window using a contralateral approach: technical feasibilities and morphometric changes of the lumbar canal and foramen. World Neurosurg 2018;117:153–61.
29. Kim SK, Kang SS, Hong YH, Park SW, Lee SC. Clinical comparison of unilateral biportal endoscopic technique versus open microdiscectomy for single-level lumbar discectomy: a multicenter, retrospective analysis. J Orthop Surg Res 2018;13:22.
30. Lin GX, Huang P, Kotheeranurak V, Park CW, Heo DH, Park CK, et al. A systematic review of unilateral biportal endoscopic spinal surgery: preliminary clinical results and complications. World Neurosurg 2019;125:425–32.
31. Li YS, Chen CM, Hsu CJ, Yao ZK. Complications of unilateral biportal endoscopic lumbar discectomy: a systematic review. World Neurosurg 2022;168:359–68.e2.
32. So JY, Park JY. Comparison of postoperative bone healing in patients with unilateral biportal endoscopic lumbar discectomy and microscopic lumbar discectomy. J Minim Invasive Spine Surg Tech 2023;8(Suppl 1):S29–38.
33. Son SK. The basic and concepts of unilateral biportal endoscopy. In: Heo DH, Park CW, Son SK, Eun JK, editors. Unilateral biportal endoscopic spine surgery basic and advanced technique textbook. Singapore: Springer; 2022. p. 9–19.
34. Wu PH. Early career challenges in setting up an endoscopic spine surgery practice. World Neurosurg 2020;144:264–9.
35. Lee CH, Choi M, Ryu DS, Choi I, Kim CH, Kim HS, et al. Efficacy and safety of full-endoscopic decompression via interlaminar approach for central or lateral recess spinal stenosis of the lumbar spine: a meta-analysis. Spine (Phila Pa 1976) 2018;43:1756–64.
36. Kim CH, Chung CK, Woo JW. Surgical outcome of percutaneous endoscopic interlaminar lumbar discectomy for highly migrated disk herniation. Clin Spine Surg 2016;29:E259–66.
37. Choi KC, Park CK. Percutaneous endoscopic lumbar discectomy for L5-S1 disc herniation: consideration of the relation between the iliac crest and L5-S1 disc. Pain Physician 2016;19:E301–8.
|
|
|||||||||||||||||||||||||||||||||||||