AbstractThis article provides a comprehensive examination of Bertolotti syndrome (BS), a disorder characterized by back pain due to a lumbosacral transitional vertebra, to facilitate surgical decision-making by exploring various surgical options, including the innovative approach of endoscopic spine surgery. A review of existing literature and studies on BS published until December 2023 was undertaken, utilizing databases such as PubMed and Google Scholar to identify relevant information. The review offers an integrated overview of the essential knowledge of BS and a comprehensive range of surgical treatments. Symptomatic BS can manifest as pain originating from pseudoarticulation and the facet joints, discs, adjacent segments, and the L5 root, indicating a diverse distribution of pain sources. Furthermore, various surgical strategies are tailored to the specific origin of pain, including pseudoarticulation resection, transverse processectomy, decompression, nerve root decompression, fusion, and endoscopic spine surgery. For individuals with BS contemplating surgical solutions, performing a detailed assessment of symptoms and physical evaluations is imperative to accurately identify the origin of the pain. The choice of a surgical strategy must be meticulously customized according to the identified source of pain, guaranteeing a tailored and efficacious treatment for each patient.
INTRODUCTIONBertolotti syndrome (BS) is defined as low back pain arising from the presence of a lumbosacral transitional vertebra (LSTV). The LSTV possesses an extensive transverse process (TP) that is pseudoarticulated or fused with the sacrum or ilium. When the TP of the lumbar vertebra enlarges, it can cause disc-induced pain and restrict mobility [1].
The clinical symptoms of BS are complex because they can range from being entirely asymptomatic to exhibiting numerous nonspecific symptoms. Complete asymptomatic cases are relatively rare, occurring in 13% of cases, and symptoms can arise from scoliosis joint arthropathy or strain in muscles such as the quadratus lumborum and iliopsoas [2]. Additionally, neurological symptoms may occur due to nerve compression from disc pressure caused by the deformation of the transitional vertebra [3]. Symptoms associated with each of these causes require different treatments.
The diagnosis of BS is made through clinical symptoms and radiographic examinations, identifying the syndrome as caused by LSTV. According to the literature, the prevalence of this syndrome widely varies, between 4%–35%, and its similarity to other diseases presenting with lower back pain may result in misdiagnosis. Moreover, the clinical symptoms of BS often do not correlate with radiographic findings, complicating radiological differentiation [4].
The optimal treatment method for BS is still under investigation and remains a topic of debate. Initially, treatment includes conservative management, local injections, radiofrequency ablation, and surgery [5]. Conservative treatments, including physical therapy and pharmacological treatment with nonsteroidal anti-inflammatory drugs, are recommended at the outset. If conservative treatment fails to provide relief, further interventions such as local injections, steroid injections, and surgical resection or fusion may be considered. Although many treatable approaches for BS exist, standardized treatment protocols and management strategies remain lacking. Therefore, identifying the mechanism and cause of pain is crucial in treating BS.
This review addresses the overall understanding of LSTV and the current surgical treatments. It focuses on establishing strategies for surgical intervention tailored to the various pain patterns that can arise from LSTV, presenting various methods to this end. This review highlights the importance of compiling all reported endoscopic surgeries for LSTV that consider their advancements.
PREVALENCELSTV possess a broad estimated prevalence range in the general population, from 4% to 36%, with an overall average of 12.3% [6]. The prevalence of LSTV is generally higher in men than in women, with some studies indicating it to be at least twice as high. Among the forms of LSTV, sacralization of L5 is more common in men, whereas accessory L5–S1 articulation and S1 lumbar articulation are more frequent in women [7]. The occurrence of LSTV in families with an increased incidence suggests a genetic factor, with the HOX10/HOX11 genes impacting the axial patterning of the lumbar and sacral vertebrae. Mutations in these genes could play a role in the formation of LSTV [8].
Although LSTV has a high prevalence in the overall population, most cases are asymptomatic, and whether LSTV is truly a cause of lower back pain remains controversial. Castellvi posited that the pain derives from abnormalities in the lumbar region, while others have argued that the severity of pain and backache is unrelated to LSTV [9,10]. Tini et al. [11] found no significant difference in the prevalence of LSTV between patients with and without lower back pain, concluding that LSTV may predict LBP. However, the presence of LBP does not necessarily predict the existence of LSTV. This uncertainty regarding the association between lower back pain and LSTV complicates the determination of the actual incidence rate among patients with BS. Quinlan et al. [12], in a study of consecutive magnetic resonance imaging (MRI) scans of 769 patients with lower back pain, found that 11.4% of those under 30 had LSTV, with an average age of 32.5 years among patients with LSTV. Understanding that LSTV affects a considerable proportion of the younger population is important.
PATHOPHYSIOLOGYThe symptoms of BS are nonspecific. Despite ongoing debate over the past century regarding the association between LSTV and lower back pain, numerous studies have pondered how the presence of LSTV can induce lower back pain. Several theories include: (1) pain secondary to arthritic changes of the pathological joint [13]; (2) pain related to accelerated disc degeneration at the level just above the LSTV [14]; (3) contralateral facetogenic pain due to abnormal stress placed on the contralateral facet joint [15]; (4) sacroiliac (SI) joint pain due to abnormal stress loading [16]; (5) impingement of the nerve root at the extraforaminal zone caused by the anomalous joint [17]. Therefore, BS is considered a multifactorial disorder, and its association with lower back pain cannot be simplified to a single pathology.
First, let us examine the anatomical impact of LSTV on our spine. The sacrum, which supports the lower part of the spine, assists in weight distribution toward the SI joint [18]. A decrease in the height of the sacrum significantly reduces the contact surface area between the sacrum and ilium, complicating the weight distribution role of the SI joint [19]. To compensate for the reduced SI joint surface area in some LSTV cases, L5 sacralization may occur in certain instances. The reduced iliolumbar ligament in these patients can arise from decreased lumbar motion due to the pseudoarticulation or fusion of LSTV. The concomitant weakening of the iliolumbar ligament and the reduced movement at LSTV can contribute to the adjacent segment instability commonly experienced by these patients [19]. Consequently, the increased loading on a relatively small sacral surface area by the large L5 TP decreases the movement at the L5-S1 junction and increases the joint mobility above it. This exacerbates disc herniation and facet arthrosis, inducing pain and leading to asymmetry in spinal movement [20].
Furthermore, in cases with LSTV, nerve root impingement has a prevalence of 13%, and up to 70% of patients with this lesion may exhibit symptoms [21]. The incomplete fusion between the L5 TP and the sacral ala and its micromotion can lead to the development of radiculopathy in patients with BS, causing extraforaminal stenosis that leads to nerve root entrapment and radiculopathy in patients with LSTV [22,23].
CLASSIFICATION OF LSTVThe LSTV was classified into 4 types by Castellvi et al. [14] in 1984, with each type (I–IV) further denoted as "a" (unilateral) or "b" (bilateral) (Figure 1). Type I signifies a TP of L5 with a width of 19 mm or more, either unilateral (Ia) or bilateral (Ib). Type II represents an extended TP that forms a "pseudoarticulation" with the sacral ala (IIa or IIb), indicating incomplete sacralization (from L5) or lumbarization (from S1). The type III classification indicates complete fusion between the TP and the alar, denoting complete sacralization of L5 or lumbarization of S1. Type IV describes a condition where one side is type IIa and the other is type IIIa [14]. Among the Castellvi classifications, type Ia is the most common, with types I and II accounting for approximately 40% of all LSTV occurrences, respectively [24]. However, the reliability of identifying morphological anomalies in the Castellvi classification is not high, with a sensitivity of 76%–84% and an accuracy of 53%–58%, leading to the proposal of several classifications for BS.
O’Driscoll et al. [25] used sagittal MRI to classify BS into 4 types based on the morphology of the S1–2 disc and the degree of lumbarization of the S1 segment. This resulted in O’Driscoll classification of sacral morphology: type 1 with no disc material between S1 and S2; type 2 with a small disc that does not extend the anteroposterior (AP) diameter of the sacrum; type 3 with a well-formed disc extending the entire sacral AP diameter; and type 4, which has the features of type 3; nevertheless, it also includes squaring of the upper sacral border. A correlation was found between type 4 S1–2 disc and types III, VI in the Castellvi classification.
The Onyiuke Grading Scale, a new grading system, classifies BS into 4 types based on the location, severity, and characteristics of the pain, focusing on clinical symptoms and less on imaging results [26]. Regarding the Jenkins classification, this new description of LSTV anatomy bases itself on the concept of a reduced gap between the TP and the sacrum as the primary cause of BS rather than disc herniation [27].
DIAGNOSIS1. Simple RadiographTraditional radiography is well documented for its utility in diagnosing and classifying LSTV. AP and lateral films allow evaluation of the spine under axial load while requiring minimal time, financial cost, and radiation exposure to the patient. An AP radiograph taken at a 30° cranial angle, known as a Ferguson radiograph, has traditionally been the standard for successfully identifying LSTV. General radiographs of the lumbosacral region demonstrate 76%–84% effectiveness in detecting LSTV. Ferguson radiographs of the lumbosacral region (AP radiographs with a 30° cranial angle) show higher sensitivity in detecting LSTV [28].
2. MRI/Computed TomographyHigh-resolution imaging increases the cost and potential radiation exposure to patients; however, it provides crucial information for accurately examining BS. Computed tomography (CT) and MRI imaging are more accurate in diagnosing and classifying LSTV than conventional radiographs, offering additional diagnostic information on adjacent areas, discs, or neurogenic pathology.
CT scans are advantageous for defining bone structures, osteophytes, and pseudoarticulation of the L5 TP [29]. MRI can have over 80% accuracy in diagnosing BS, with T2-weighted coronal images being most effective in diagnosing lesions known as the "far-out" syndrome, where nerve roots are impinged between the TP of L5 and the sacral alar.
3. ScintigraphyBeyond standard radiographs, CT, and MRI, bone scintigraphy may help identify potential sources of pain in patients with BS. Abnormal articulations in LSTV can lead to degenerative and metabolic changes, possibly related to the patient's pain. These changes show increased absorption in bone scintigraphy, with significantly increased absorption observed in symptomatic patients with degenerative changes on single-photon emission computed tomography (SPECT) images [30]. Radiography and injections aside, bone scintigraphy tests such as SPECT/CT and positron emission tomography/CT have shown potential in identifying the source of pain in patients with BS.
4. Slit-Beam Digital Radiography SystemThe slit-beam digital radiography system, a new radiographic method that emits low doses of radiation, provides accurate 3-dimensional (3D) images of spinal anatomy, which is significant for differentiating and classifying BS. It captures upright orthogonal images and reconstructs 3D images of the skeletal structure (specifically the spine and pelvis), which is useful in determining the relationship between anatomical regions and adjacent segments [31,32]. Despite advancements in imaging techniques, diagnosing BS remains challenging. Differential diagnosis for low back pain is extensive, including myofascial pain, SI pain, fractures (including spondylolysis), spondylolisthesis, scoliosis, disc degeneration/herniation, infection, and malignancy [5,12]. This wide range of differential diagnosis can lead to delayed or missed diagnoses.
SURGICAL PLANNING CONSIDERATIONSConservative treatments include activity modification, pharmacologic therapy, physical therapy, and interventional therapy. Patients with BS should first undergo conservative treatment before proceeding to invasive treatments such as steroid and anesthetic injections and surgical interventions like removal of LSTV pseudoarticulation, decompression, or fusion. Localized injections can identify the primary source of the patient's pain, enabling targeted surgical treatment and preventing total removal. The degree of pain relief following local injections provides valuable information for guiding surgical treatment if the pain resolves. Surgical intervention can be considered when no response is available for conservative treatment.
Pain caused by LSTV can originate from various lesions. Pain may arise directly from the pseudoarticulation itself. However, it can also be due to asymmetric segmental motion between LSTV and the sacrum, exacerbating weight load on the opposite facet joint, leading to facet arthritis as a source of pain, or increased weight load on the adjacent segment above due to reduced segmental motion [33-36]. In cases where discogenic pain exists concurrently at the LSTV level, performing resection of pseudoarticulation alone may not meet expectations for pain improvement or may worsen discogenic pain due to increased intersegmental movement post-resection. Therefore, in cases with accompanying disc pathology or when pain is associated with increased instability and mobility of the segment, fusion, which can provide long-term stability, may be a better choice than resection [37,38]. Surgeons must be aware of all possibilities that the pain source in patients with symptomatic LSTV may be localized to the pseudoarticulation or may reside in various other lesions, and even possibly more significant than the pseudoarticulation itself, and meticulously plan the surgery after verification. Figure 2 presents a diagnostic and therapeutic diagram of symptomatic LSTVs.
SURGICAL OPTIONSSurgical treatments for BS including resection, nerve root decompression, and fusion using microscopic techniques have been reported by numerous authors. Endoscopic spinal surgery techniques have improved recently, and there have been several reports of surgical attempts to address BS using them. Surgical methods can be categorized as traditional microscopic and endoscopic techniques.
1. Microscopic Surgery1) ResectionAmong the subtypes of LSTV, type II has been reported to have the highest prevalence of low back pain at 73% [38]. According to various reports, the primary candidates for surgical resection of symptomatic BS are those with type II LSTV, which form pseudoarticulations, rather than other types that do not form pseudoarticulations or are already in a state of complete bony union [39,40]. If the pain generator is diagnosed as being localized to the pseudoarticulation, resection becomes the most effective surgical option. The excision of the anomalous connection between the LSTV and the sacrum can mitigate asymmetrical or diminished segmental motion, with potential benefits in alleviating adjacent segment or contralateral facet arthritis to some degree [15]. However, discogenic pain may worsen due to increased segmental motion of the LSTV after resection. Therefore, surgeons must plan the surgery by predicting the clinical outcomes based on the changes in the distribution of mechanical stress around the LSTV before and after resection. In cases accompanied by far-out syndrome, the surgical plan should include root decompression, which can be performed simultaneously with the resection. To prevent misdiagnosis, a diagnostic block of the pseudoarticulation is recommended before surgery [34]. Most authors reporting on the resection of LSTV have performed a preoperative diagnostic block [41]. Nonfusion surgeries reported to date for removing pain originating from pseudoarticulation can be categorized into resection of pseudoarticulation, TP resection, and anterior approach technique.
(1) Pseudoarticulation resectionThe resection of the pseudoarticulation technique directly removes the pathological tissue causing pain and is a standard method that can disconnect the mechanical impact on adjacent joints. Using AP fluoroscopic imaging to locate the TPs, sacrum, and articular processes, a 2.5- to 4-cm vertical incision is made approximately 4 cm lateral from the midline, directly above the articular process. The fascia is sharply opened, and muscle/ligament attachments are removed to expose the TP, pseudoarticulation, and sacral alar. High-speed drills are then used to remove the pseudoarticulation. A tubular retractor may be used to minimize tissue damage [40-44], although its use may limit surgical visibility, making the surgery more challenging and possibly leading to inadequate decompression [41]. Care should be taken when selecting the incision site as the iliac crest may obstruct the surgical trajectory, with some authors reporting resection of part of the left posterior iliac crest to access the pseudo articulation [43]. Pseudoarthrectomy can present challenges due to the potentially wide and irregular anatomical shapes, leading to anatomic misorientation within the operative field, which can be particularly challenging for surgeons with less experience in BS resection surgery. This may result in unnecessary resection of normal tissue or insufficient resection of the target lesion. Thus, confirming the most ventrolateral margin of the pseudoarticulation is recommended before beginning resection with high-speed drilling [45]. Navigation for stereotactic localization of the pseudojoint has been reported as a viable complementary method, or repeated verification with a C-arm is recommended if navigation is unavailable [42,44,46].
(2) Transverse processectomyAnother reported method for BS resection surgery is transverse processectomy. This method differs from the previously described technique as it only resects the TP without directly removing the pseudoarticulation. The therapeutic principle is explained as blocking the path through which mechanical stress from the spine is transferred to the pseudoarticulation [47]. After making a skin incision approximately 2 to 4 cm from the midline, 3.5 to 4 cm laterally, access is gained between the multifidus and longissimus muscles to expose the base of the L5 TP and the upper part of the sacral ala. To avoid damaging the iliolumbar ligament and pseudoarticulation, the lateral end of the L5 TP is not exposed, and space is created by accessing only its base. The upper and lower edges of the L5 TP are palpated, and a high-speed drill is used to cut the base of the L5 TP, maintaining at least a 0.5 cm cutting gap to prevent rejoining. A tubular retractor may be used based on the surgeon's preference. The resected L5 TP can be removed en bloc or in pieces using a pituitary rongeur. Bone wax can be applied to the cut surface of the sacrum to prevent excessive bleeding. Compared to pseudoarthrectomy, transverse processectomy is less invasive and has a shorter operation time, but it is less effective at relieving pain from the pseudoarticulation [47]. The therapeutic principle of transverse processectomy is not fully understood, and it is assumed to reduce the mechanical load on the pseudoarticulation by blocking the path of stress transmission from the spine. However, there is a risk of rejoining or non-union of the TP, leading to the recurrence of symptoms. Therefore, careful patient selection is essential, and this technique is recommended for patients with mild symptoms or those who are not suitable candidates for more invasive procedures.
(3) Anterior approach techniqueThe anterior approach technique involves accessing the pseudoarticulation through an anterior incision, allowing for direct visualization and resection of the pseudoarticulation without disturbing the posterior structures of the spine. This technique is less commonly reported in the literature. It is typically reserved for cases where the pseudoarticulation is anteriorly positioned or when there is a need to address other anterior spinal pathologies simultaneously. The approach requires careful planning and understanding of the vascular and visceral anatomy to avoid complications. The anterior approach may offer advantages in reduced muscle dissection and potentially quicker recovery times, but it also carries risks associated with abdominal surgery, such as injury to the great vessels, ureter, or intestines. This technique is considered for patients with specific anatomical considerations or when a combined anterior-posterior approach is necessary to address complex spinal pathologies in addition to BS. Due to the complexity and potential risks, it is typically performed by surgeons with expertise in anterior spinal surgery and collaboration with vascular or general surgeons as needed.
2) Nerve root decompressionThere are cases where L5 radiculopathy accompanies far-out syndrome. The L5 nerve root is lateral to the L5–S1 disc, lateral to the L5 TP, and medial to the pseudoarticulation. The L5 root is compressed between the impingement of the L5 TP and the sacral ala, causing symptoms [48]. Therefore, the main surgical process to decompress the L5 nerve root involves expanding the pathway of the L5 root by partially resecting the bony structure surrounding it, namely the L5 TP, sacral ala, and pseudoarticulation, which can mostly be achieved through a posterior approach [23,49,50]. Abe et al. [51] have reported a case where neural decompression was performed using an anterior approach for a patient complaining of radiating pain due to far-out syndrome caused by LSTV. This patient underwent neural decompression through an extraperitoneal approach due to prominent bony spur formation in the anterior exit zone of the lateral wall of the L5 root foramen, reporting good clinical outcomes. However, this case has limitations in that symptoms took extended period to improve after surgery, and the possibility of improvement due to natural progression rather than surgical intervention cannot be entirely ruled out. They also advised that reducing the tightness of the root due to the surgical position could decrease the damage to the root during surgery.
3) FusionThe fusion technique has traditionally been adopted in most of the literature reported so far, utilizing pedicle screwing and intersegmental posterolateral fusion, and in some cases, introduced for symptomatic BS using a tubular retractor system [52]. However, it seems no additional techniques are needed because it is BS. Fusion is more invasive compared to resection, with concerns of higher surgical complications and, in the long term, known to induce adjacent segment degeneration, potentially causing other problems. Nonetheless, there are reasons why fusion can sometimes be a more viable option in the surgical treatment of BS.
Firstly, in cases where discogenic pain exists simultaneously at the LSTV level. In such cases, resection alone may not satisfactorily improve symptoms, and discogenic pain might worsen due to increased intersegmental movement after resection. Dhanjani et al. [53] reported long-term good outcomes from classical fusion surgery on a 13-year-old female patient with symptomatic Castellvi type IIa BS, who showed extensive TP bridging, considering the disc of that segment as a potential source of pain.
Another crucial point not to be overlooked in deciding on fusion is confirming the existence of pain generators at the adjacent segment above LSTV, L4–5, and the SI joint. If symptomatic disc degeneration, facet arthritis, spondylolysis, or SI arthritis were not identified before surgery and existed in adjacent segments, physical stress due to weight-bearing after fusion surgery for some types of BS could exacerbate persistent pain in these areas. It is worth noting that compared to the non-LSTV population, LSTV can have reduced intersegmental mobility, potentially leading to compensatory hypermobility in adjacent segments [4], which has been reported as a primary cause of disc degeneration [36]. Jenikar et al. [54], in their cohort observational study comparing patients with and without LSTV, reported that LSTV results in more degenerative changes in the adjacent upper segment and additionally. Therefore, if pain generators are diagnosed in the upper segment or SIJ while planning fusion for the LSTV segment, it may be necessary to plan for multilevel fusion, including those segments, with surgical decisions considering the risk-benefit.
Mikula et al. [55] reported that comparing the clinical efficacy of a group that underwent resection of pseudoarticulation with a group that underwent fusion for symptomatic BS, fusion showed superior pain improvement in both short-term outcomes within 6 months and long-term outcomes beyond 12 months. Notably, the rate of maintained pain improvement until the long-term outcome was statistically significantly different, with 28% in the resection group compared to 78% in the fusion group, which is worth considering.
2. Endoscopic Surgery1) Full endoscopyWith the advancement of endoscopic spinal surgery techniques, various attempts at surgical interventions for BS have been reported. Replacing traditional surgical methods with endoscopic procedures, such as pseudoarticulation or transverse processectomy and root decompression, allows for less invasive operations that perform most of the surgical process similarly, with clinical effects comparable to conventional methods. The endoscopy techniques reported for BS to date are summarized in Table 1.
(1) Nerve root decompression for treating far-out syndromePaudel et al. [56] reported the results of performing a full endoscopy on 3 patients diagnosed with far-out syndrome caused by LSTV, who did not respond to conservative treatment. This report is the first of its kind regarding full endoscopy for BS. The patients were diagnosed preoperatively with compression of the L5 root between the pseudoarticulation at the TP and the sacral alar, causing sciatica. The authors introduced methods of achieving L5 root decompression by removing the distal part of the TP with high-speed burr drilling through a direct dorsal approach to the endoscope's working area and by resecting parts of the TP or pseudoarticulation similar to percutaneous endoscopic transforaminal lumbar discectomy. They noted that patients showed good clinical outcomes after more than a year postsurgery, suggesting that this direct target-oriented surgery, which minimizes soft tissue injury compared to classical methods using microscopes or tubular retractors, is advantageous for postoperative recovery. Specifically, preserving the iliolumbar ligament, crucial for the stability of the lumbosacral junction, was highlighted as a benefit.
(2) Pseudoarticulation resectionThe first report of performing resection of pseudoarticulation using full endoscopy for symptomatic BS was in 2021, with Stein et al. [57] reporting a similar surgical method 2 years later. Wu et al. [45] provided a detailed description of the surgical technique in a technical note. They described entering the endoscopy to the target area through a 1-cm skin and fascia incision at the midpoint of the pseudoarticulation under fluoroscopic guidance. They proceeded with drilling from the ventrolateral margin of the pseudoarticulation articulating with the highest part of the sacral ala (PH point) in a superficial to deep fashion towards the dorsal medioinferior margin adjacent to the superior articular process (MS point), followed by L5 root decompression. Wu emphasized the importance of preoperative MRI to check the course of the L5 nerve root, secure identification of the PH point to prevent drilling-induced nerve injury and retroperitoneal space penetration and maintain a 9-mm gap between the dysplastic TP and sacral alar to prevent recurrence of fusion. Stein et al. [57] mentioned that extensive resection provides superior pain relief.
(3) Transverse processectomyIn 2019, a report was published on performing transverse processectomy using full endoscopy for symptomatic BS. Yoo et al. [58] reported on a 64-year-old female patient with left leg pain, initially diagnosed with foraminal stenosis at L5–S1 and treated with foraminotomy using full endoscopy without symptom improvement. Subsequent identification of pseudoarticulation as the pain generator through a pseudoarticulation block led to symptom improvement through a second surgery. The authors noted that this method, which involved drilling the base of L5's TP with a high-speed burr, replicated a technique reported by Ju et al. [47] in 2017 using microscopic surgery. This surgical approach can block the pathway of mechanical stress from body weight on the pseudoarticulation and simultaneously perform L5 root decompression in cases of far-out syndrome. The anatomical recognition of the TP being relatively straightforward in the operative field facilitates the surgery and identification of the L5 root, thus combining the advantages of the reported transverse processectomy method with those of full endoscopy.
2) Unilateral biportal endoscopyIn 2019, Heo et al. [59] were the first to report on unilateral biportal endoscopy (UBE) conducted for radiculopathy caused by far-out syndrome. This is the only report using UBE for symptomatic BS, including clinical outcomes for 14 cases and a technical note. According to the surgical procedures of authors, the surgery was performed under general or epidural anesthesia. Two skin incisions were made 1 cm lateral to the lateral border of the L5–S1 pedicle and 1 cm above and below the midpoint of the foramen, after which an endoscopic channel and a working channel were formed at each incision. The decompressive procedure began with the partial drilling of the lower portion of the TP and the lateral portion of the isthmus and the facet wall, exposing the foraminal part of the L5 root and continued by following the course of the nerve root. Decompression was performed from the superior portion of the ala medially to laterally, drilling out the pseudoarticulation while simultaneously decompressing the root.
The authors presented clinical outcomes after an average follow-up of 11 months, stating that UBE approaches demonstrated shorter operation times and less blood loss while minimizing damage to posterior muscle and ligamentous structures. They also highlighted the advantages of reduced postoperative pain and easier recovery. However, they noted disadvantages, including retroperitoneal fluid collection due to irrigation fluid, the possibility of incomplete decompression, and the steep learning curve of endoscopy. Specifically, they emphasized the need to explore and remove any concomitant extraforaminal disc herniation after decompression around the pseudoarticulation, as it may be associated with a sudden aggravation of pain. The surgical process introduced by Heo et al. [59], which allows for L5 root decompression from the foraminal to the extraforaminal area and involves verifying the L5 root from proximal to direct vision sequentially to lateral decompression, is considered relatively safer compared to methods introduced by other authors.
CONCLUSIONSurgical intervention should only be considered for symptomatic BS when conservative treatments fail. LSTV can cause symptoms in various forms, including pseudoarticulation, facet arthritis, disc degeneration, adjacent segment degeneration, and far-out syndrome. A precise presurgical investigation of the distribution of pain generators is necessary, and the appropriate surgical treatment should be chosen based on the type of pseudoarticulation. Before proceeding with surgery, careful consideration of the expected benefits of pain relief from surgical intervention is required. While endoscopic surgery for BS has been attempted numerous times and demonstrated successful outcomes, additional research and evidence are needed.
NOTESConflict of Interest CIJ and PK, are member of the Editorial Board of Journal of Minimally Invasive Spine Surgery & Technique, are the author of this article. However, they played no role whatsoever in the editorial evaluation of this article or the decision to publish it. The other authors have no conflict of interest to declare. Figure 1.Castellvi classification system of lumbosacral transitional vertebrae. Adapted from Castellvi et al. Spine 1984;9:493-5 [14], with permission of Elsevier. 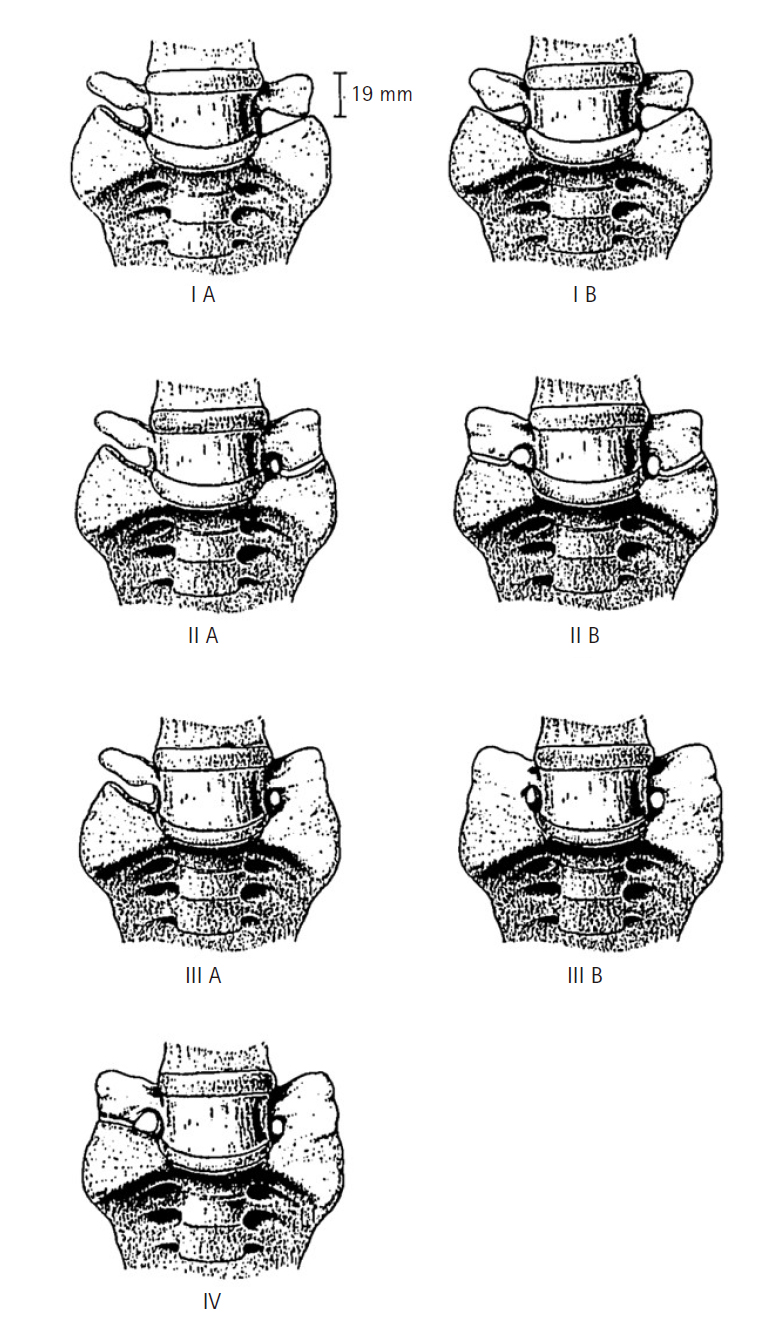
Figure 2.Flow chart for diagnosing and treating symptomatic lumbosacral transitional vertebrae. CT, computed tomography; LSTV, lumbosacral transitional vertebrae; MRI, magnetic resonance imaging. 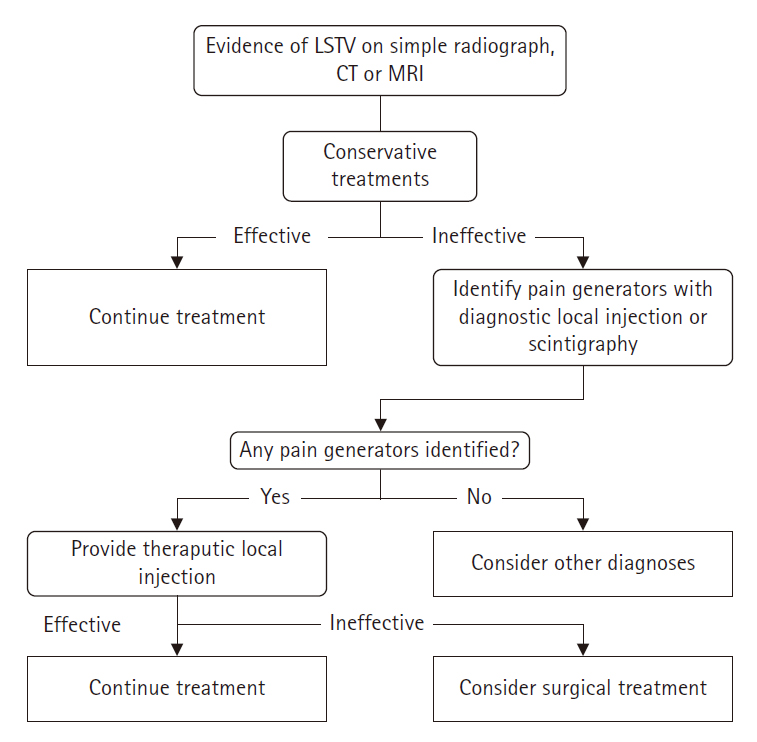
Table 1.Summary of the current literature on endoscopic spine surgery for Bertolotti syndrome
REFERENCES1. Crane J, Cragon R, O'Neill J, Berger AA, Kassem H, Sherman WF, et al. A comprehensive update of the treatment and management of Bertolotti's syndrome: a best practices review. Orthop Rev (Pavia) 2021;13:24980.
2. Kapetanakis S, Chaniotakis C, Paraskevopoulos C, Pavlidis P. An unusual case report of Bertolotti's syndrome: extraforaminal stenosis and L5 unilateral root compression (Castellvi type III an LSTV). J Orthop Case Rep 2017;7:9–12.
3. Jain A, Agarwal A, Jain S, Shamshery C. Bertolotti syndrome: a diagnostic and management dilemma for pain physicians. Korean J Pain 2013;26:368–73.
4. Golubovsky JL, Colbrunn RW, Klatte RS, Nagle TF, Briskin IN, Chakravarthy VB, et al. Development of a novel in vitro cadaveric model for analysis of biomechanics and surgical treatment of Bertolotti syndrome. Spine J 2020;20:638–56.
5. Almeida DB, Mattei TA, Sória MG, Prandini MN, Leal AG, Milano JB, et al. Transitional lumbosacral vertebrae and low back pain: diagnostic pitfalls and management of Bertolotti's syndrome. Arq Neuropsiquiatr 2009;67(2A):268–72.
6. Bron JL, van Royen BJ, Wuisman PI. The clinical significance of lumbosacral transitional anomalies. Acta Orthop Belg 2007;73:687–95.
7. Uçar D, Uçar BY, Coşar Y, Emrem K, Gümüşsuyu G, Mutlu S, et al. Retrospective cohort study of the prevalence of lumbosacral transitional vertebra in a wide and well-represented population. Arthritis 2013;2013:461425.
8. Paik NC, Lim CS, Jang HS. Numeric and morphological verification of lumbosacral segments in 8280 consecutive patients. Spine (Phila Pa 1976) 2013;38:E573–8.
9. Frymoyer JW, Newberg A, Pope MH, Wilder DG, Clements J, MacPherson B. Spine radiographs in patients with low-back pain. An epidemiological study in men. J Bone Joint Surg Am 1984;66:1048–55.
10. Otani K, Konno S, Kikuchi S. Lumbosacral transitional vertebrae and nerve-root symptoms. J Bone Joint Surg Br 2001;83:1137–40.
11. Tini PG, Wieser C, Zinn WM. The transitional vertebra of the lumbosacral spine: its radiological classification, incidence, prevalence, and clinical significance. Rheumatol Rehabil 1977;16:180–5.
12. Quinlan JF, Duke D, Eustace S. Bertolotti's syndrome. A cause of back pain in young people. J Bone Joint Surg Br 2006;88:1183–6.
13. Jönsson B, Strömqvist B, Egund N. Anomalous lumbosacral articulations and low-back pain. Evaluation and treatment. Spine (Phila Pa 1976) 1989;14:831–4.
14. Castellvi AE, Goldstein LA, Chan DP. Lumbosacral transitional vertebrae and their relationship with lumbar extradural defects. Spine (Phila Pa 1976) 1984;9:493–5.
15. Brault JS, Smith J, Currier BL. Partial lumbosacral transitional vertebra resection for contralateral facetogenic pain. Spine (Phila Pa 1976) 2001;26:226–9.
16. Reitsma AM, Schuler TC, Hasz MW, Poelstra KA. Surgical treatment of symptomatic Bertolotti's syndrome in post-fusion patients. Orthopedics 2002;25:340–2.
17. Shibayama M, Ito F, Miura Y, Nakamura S, Ikeda S, Fujiwara K. Unsuspected reason for sciatica in Bertolotti's syndrome. J Bone Joint Surg Br 2011;93:705–7.
18. Mahato NK. Relationship of sacral articular surfaces and gender with occurrence of lumbosacral transitional vertebrae. Spine J 2011;11:961–5.
19. Mahato NK. Morphological traits in sacra associated with complete and partial lumbarization of first sacral segment. Spine J 2010;10:910–5.
20. Paraskevas G, Tzaveas A, Koutras G, Natsis K. Lumbosacral transitional vertebra causing Bertolotti's syndrome: a case report and review of the literature. Cases J 2009;2:8320.
21. Porter NA, Lalam RK, Tins BJ, Tyrrell PN, Singh J, Cassar-Pullicino VN. Prevalence of extraforaminal nerve root compression below lumbosacral transitional vertebrae. Skeletal Radiol 2014;43:55–60.
22. Hashimoto M, Watanabe O, Hirano H. Extraforaminal stenosis in the lumbosacral spine. efficacy of MR imaging in the coronal plane. Acta Radiol 1996;37:610–3.
23. Kojo S, Takahashi K, Tsubakino T, Hashimoto K, Aizawa T, Tanaka Y. Lumbar radiculopathy due to Bertolotti's syndrome: alternative method to reveal the "hidden zone" - A report of two cases and review of literature. J Orthop Sci 2024;29:366–9.
24. Nardo L, Alizai H, Virayavanich W, Liu F, Hernandez A, Lynch JA, et al. Lumbosacral transitional vertebrae: association with low back pain. Radiology 2012;265:497–503.
25. O'Driscoll CM, Irwin A, Saifuddin A. Variations in morphology of the lumbosacral junction on sagittal MRI: correlation with plain radiography. Skeletal Radiol 1996;25:225–30.
26. Knopf J, Lee S, Bulsara K, Moss I, Choi D, Onyiuke H. Onyiuke grading scale: a clinical classification system for the diagnosis and management of Bertolotti syndrome. Neurochirurgie 2021;67:540–6.
27. Jenkins AL 3rd, O'Donnell J, Chung RJ, Jenkins S, Hawks C, Lazarus D, et al. Redefining the classification for Bertolotti syndrome: anatomical findings in lumbosacral transitional vertebrae guide treatment selection. World Neurosurg 2023;175:e303–13.
28. Neelakantan S, Anandarajan R, Shyam K, Philip B. Multimodality imaging in Bertolotti's syndrome: an important cause of low back pain in young adults. BMJ Case Rep 2016;2016:bcr2016217121.
29. Benvenuto P, Benvenuto N. Bertolotti's syndrome: a transitional anatomic cause of low back pain. Intern Emerg Med 2018;13:1333–4.
30. Pekindil G, Sarikaya A, Pekindil Y, Gültekin A, Kokino S. Lumbosacral transitional vertebral articulation: evaluation by planar and SPECT bone scintigraphy. Nucl Med Commun 2004;25:29–37.
31. Melhem E, Assi A, El Rachkidi R, Ghanem I. EOS(®) biplanar X-ray imaging: concept, developments, benefits, and limitations. J Child Orthop 2016;10:1–14.
32. Albano D, Messina C, Gambino A, Gurgitano M, Sciabica C, Oliveira Pavan GR, et al. Segmented lordotic angles to assess lumbosacral transitional vertebra on EOS. Eur Spine J 2020;29:2470–6.
33. Elster AD. Bertolotti's syndrome revisited. Transitional vertebrae of the lumbar spine. Spine (Phila Pa 1976) 1989;14:1373–7.
34. Avimadje M, Goupille P, Jeannou J, Gouthière C, Valat JP. Can an anomalous lumbo-sacral or lumbo-iliac articulation cause low back pain? A retrospective study of 12 cases. Rev Rhum Engl Ed 1999;66:35–9.
35. Takata Y, Sakai T, Higashino K, Goda Y, Mineta K, Sugiura K, et al. Minimally invasive microendoscopic resection of the transverse process for treatment of low back pain with Bertolotti's syndrome. Case Rep Orthop 2014;2014:613971.
36. Farshad-Amacker NA, Herzog RJ, Hughes AP, Aichmair A, Farshad M. Associations between lumbosacral transitional anatomy types and degeneration at the transitional and adjacent segments. Spine J 2015;15:1210–6.
37. Santavirta S, Tallroth K, Ylinen P, Suoranta H. Surgical treatment of Bertolotti's syndrome. follow-up of 16 patients. Arch Orthop Trauma Surg 1993;112:82–7.
38. McGrath K, Schmidt E, Rabah N, Abubakr M, Steinmetz M. Clinical assessment and management of Bertolotti syndrome: a review of the literature. Spine J 2021;21:1286–96.
39. Li Y, Lubelski D, Abdullah KG, Mroz TE, Steinmetz MP. Minimally invasive tubular resection of the anomalous transverse process in patients with Bertolotti's syndrome: presented at the 2013 Joint Spine Section Meeting: clinical article. J Neurosurg Spine 2014;20:283–90.
40. McGrath KA, Thompson NR, Fisher E, Kanasz J, Golubovsky JL, Steinmetz MP. Quality-of-life and postoperative satisfaction following pseudoarthrectomy in patients with Bertolotti syndrome. Spine J 2022;22:1292–300.
41. Chang CJ, Chiu YP, Ji HR, Chu CH, Chiu CD. Surgical interventions for Bertolotti's syndrome: case report and review of unsatisfactory cases in the literature. BMC Surg 2022;22:36.
42. Yousif S, Wood M. Minimally invasive resection of lumbosacral pseudojoint resulting in complete resolution of a lower back pain - A case report and review of Bertolotti syndrome. J Clin Neurosci 2018;51:67–8.
43. Shinonara K, Kaneko M, Ugawa R, Arataki S, Takeuchi K. The effectiveness of preoperative assessment using a patient-specific three-dimensional pseudoarticulation model for minimally invasive posterior resection in a patient with Bertolotti's syndrome: a case report. J Med Case Rep 2021;15:68.
44. Afana H, Raffat M, Figueiredo N. Surgical pitfalls in Bertolotti's syndrome management: a case report. Medicine (Baltimore) 2022;101:e32293.
45. Wu PH, Sebastian M, Kim HS, Heng GTY. How I do it? Uniportal full endoscopic pseudoarthrosis release of left L5/S1 Bertolotti's syndrome under intraoperative computer tomographic guidance in an ambulatory setting. Acta Neurochir (Wien) 2021;163:2789–95.
46. Babu H, Lagman C, Kim TT, Grode M, Johnson JP, Drazin D. Intraoperative navigation-guided resection of anomalous transverse processes in patients with Bertolotti's syndrome. Surg Neurol Int 2017;8:236.
47. Ju CI, Kim SW, Kim JG, Lee SM, Shin H, Lee HY. Decompressive L5 transverse processectomy for Bertolotti's syndrome: a preliminary study. Pain Physician 2017;20:E923–32.
48. Wiltse LL, Guyer RD, Spencer CW, Glenn WV, Porter IS. Alar transverse process impingement of the L5 spinal nerve: the far-out syndrome. Spine (Phila Pa 1976) 1984;9:31–41.
49. Ichihara K, Taguchi T, Hashida T, Ochi Y, Murakami T, Kawai S. The treatment of far-out foraminal stenosis below a lumbosacral transitional vertebra: a report of two cases. J Spinal Disord Tech 2004;17:154–7.
50. Miyoshi Y, Yasuhara T, Date I. Posterior decompression of far-out foraminal stenosis caused by a lumbosacral transitional vertebra--case report. Neurol Med Chir (Tokyo) 2011;51:153–6.
51. Abe E, Sato K, Shimada Y, Okada K, Yan K, Mizutani Y. Anterior decompression of foraminal stenosis below a lumbosacral transitional vertebra. A case report. Spine (Phila Pa 1976) 1997;22:823–6.
52. Adams R, Herrera-Nicol S, Jenkins AL 3rd. Surgical treatment of a rare presentation of Bertolotti's syndrome from Castellvi type IV lumbosacral transitional vertebra: case report and review of the literature. J Neurol Surg Rep 2018;79:e70–4.
53. Dhanjani S, Altaleb M, Margalit A, Puvanesarajah V, Jain A. Pediatric back pain associated with Bertolotti syndrome: a report of 3 cases with varying treatment strategies. JBJS Case Connect 2021 11(4):doi: 10.2106/JBJS.CC.21.00068.
54. Jenikar P, Cattamanchi VC, Kumar R, Mallikarjunappa B. Lumbosacral transitional vertebra: Prevalence and its impact on transitional and adjacent lumbar discs. IOSR J Dental Med Sci 2019;18:1–8.
55. Mikula AL, Lakomkin N, Ransom RC, Flanigan PM, Waksdahl LA, Pennington Z, et al. Operative treatment of Bertolotti syndrome: resection versus fusion. World Neurosurg 2022;165:e311–6.
56. Paudel B, Kim HS, Jang JS, Choi JH, Chung SK, Lee JS, et al. Percutaneous full endoscopic treatment of bertolotti syndrome: a report of three cases with technical note. J Neurol Surg A Cent Eur Neurosurg 2017;78:566–71.
57. Stein E, Panjeton GD, Kumar S. Endoscopic resection of pseudoarticulation as a treatment for Bertolotti's syndrome. Cureus 2023;15:e33397.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||